Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum - PubMed (original) (raw)
Tea polyphenols inhibit the growth and virulence properties of Fusobacterium nucleatum
Amel Ben Lagha et al. Sci Rep. 2017.
Abstract
Fusobacterium nucleatum plays a key role in creating the pathogenic subgingival biofilm that initiates destructive periodontitis. It is also a common resident of the human gastrointestinal tract and has been associated with inflammatory bowel disease. The aim of the present study was to investigate the effects of green and black tea extracts as well as two of their bioactive components, EGCG and theaflavins, on the growth and virulence properties of F. nucleatum. The tea extracts and components displayed various degrees of antibacterial activity that may involve damage to the bacterial cell membrane and the chelation of iron. They also prevented biofilm formation by F. nucleatum at concentrations that did not interfere with bacterial growth. In addition, the treatment of a pre-formed F. nucleatum biofilm with the green tea extract and EGCG caused a time-dependent decrease in biofilm viability. The green and black tea extracts, EGCG, and theaflavins decreased the adherence of F. nucleatum to oral epithelial cells and matrix proteins. Moreover, these tea components also attenuated F. nucleatum-mediated hemolysis and hydrogen sulfide production, two other virulence factors expressed by this bacterium. In summary, this study showed that tea polyphenols may be of interest for treating F. nucleatum-associated disorders.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
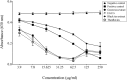
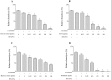
Figure 1. Iron-chelating activity of the green tea extract, black tea extract, EGCG, and theaflavins determined using a siderophore colorimetric assay.
A decrease in A630 occurs when a strong chelator removes the iron from the chrome azurol sulfate dye. Ferrichrome, a siderophore produced by U. sphaerogena, was used as positive control. Results are expressed as the means ± SD of triplicate assays from two independent experiments. All values are significantly different from the negative control (p < 0.01).
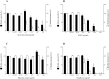
Figure 2. Effects of the green tea extract, black tea extract, EGCG, and theaflavins on the growth of and biofilm formation by F. nucleatum.
A value of 100% was assigned to growth and biofilm formation obtained with F. nucleatum in the absence of tea polyphenols. Results are expressed as the means ± SD of triplicate assays from three independent experiments. *Significantly different from the control (p < 0.01).
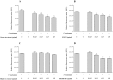
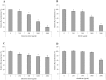
Figure 3. Effects of the green tea extract, black tea extract, EGCG, and theaflavins on the adherence of F. nucleatum to oral epithelial cells.
Results are expressed as the means ± SD of triplicate assays from two independent experiments. *Significant decrease (p < 0.01) compared with the untreated control.
Figure 4. Effects of the green tea extract, black tea extract, EGCG, and theaflavins on the adherence of F. nucleatum to extracellular matrix proteins (Matrigel®).
Results are expressed as the means ± SD of triplicate assays from two independent experiments. *Significant decrease (p < 0.01) compared with the untreated control.
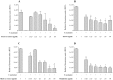
Figure 5. Effects of the green tea extract, black tea extract, EGCG, and theaflavins on the hemolytic activity of F. nucleatum.
SDS (10%) was used as positive control to induce complete lysis of sheep erythrocytes. A value of 100% was assigned to the hemolysis by F. nucleatum in the absence of tea polyphenols. Results are expressed as the means ± SD of triplicate assays from three independent experiments. *Significantly different from the control (p < 0.01).
Figure 6. Effects of the green tea extract, black tea extract, EGCG, and theaflavins on H2S production by F. nucleatum.
A value of 100% was assigned to H2S production by F. nucleatum in the absence of tea polyphenols. Results are expressed as the means ± SD of triplicate assays from three independent experiments. *Significantly different from the control (p < 0.01).
Similar articles
- Wild Blueberry (Vaccinium angustifolium Ait.) Polyphenols Target Fusobacterium nucleatum and the Host Inflammatory Response: Potential Innovative Molecules for Treating Periodontal Diseases.
Ben Lagha A, Dudonné S, Desjardins Y, Grenier D. Ben Lagha A, et al. J Agric Food Chem. 2015 Aug 12;63(31):6999-7008. doi: 10.1021/acs.jafc.5b01525. Epub 2015 Aug 4. J Agric Food Chem. 2015. PMID: 26207764 - Green tea extract and its major constituent epigallocatechin-3-gallate inhibit growth and halitosis-related properties of Solobacterium moorei.
Morin MP, Bedran TB, Fournier-Larente J, Haas B, Azelmat J, Grenier D. Morin MP, et al. BMC Complement Altern Med. 2015 Mar 10;15:48. doi: 10.1186/s12906-015-0557-z. BMC Complement Altern Med. 2015. PMID: 25880992 Free PMC article. - Fusobacterium nucleatum: an emerging gut pathogen?
Allen-Vercoe E, Strauss J, Chadee K. Allen-Vercoe E, et al. Gut Microbes. 2011 Sep 1;2(5):294-8. doi: 10.4161/gmic.2.5.18603. Epub 2011 Sep 1. Gut Microbes. 2011. PMID: 22067936 Review. - Mixed oral biofilms are controlled by the interspecies interactions of Fusobacterium nucleatum.
Valadbeigi H, Khoshnood S, Negahdari B, Maleki A, Mohammadinejat M, Haddadi MH. Valadbeigi H, et al. Oral Dis. 2024 Sep;30(6):3582-3590. doi: 10.1111/odi.14822. Epub 2023 Nov 27. Oral Dis. 2024. PMID: 38009960 Review.
Cited by
- Effects of Green Tea Extract Epigallocatechin-3-Gallate on Oral Diseases: A Narrative Review.
Li Y, Cheng L, Li M. Li Y, et al. Pathogens. 2024 Jul 29;13(8):634. doi: 10.3390/pathogens13080634. Pathogens. 2024. PMID: 39204235 Free PMC article. Review. - Lavandula angustifolia Essential Oil Inhibits the Ability of Fusobacterium nucleatum to Produce Volatile Sulfide Compounds, a Key Components in Oral Malodor.
Rosner O, Livne S, Bsharat M, Dviker S, Jeffet U, Matalon S, Sterer N. Rosner O, et al. Molecules. 2024 Jun 23;29(13):2982. doi: 10.3390/molecules29132982. Molecules. 2024. PMID: 38998934 Free PMC article. - Dencichine attenuates the virulence of Fusobacterium nucleatum by targeting hydrogen sulfide-producing enzyme.
Wang M, Chu W. Wang M, et al. Int Microbiol. 2024 May 25. doi: 10.1007/s10123-024-00539-1. Online ahead of print. Int Microbiol. 2024. PMID: 38789725 - Advances of Oxidative Stress Impact in Periodontitis: Biomarkers and Effective Targeting Options.
Pouliou C, Piperi C. Pouliou C, et al. Curr Med Chem. 2024;31(38):6187-6203. doi: 10.2174/0109298673297545240507091410. Curr Med Chem. 2024. PMID: 38726786 Review. - Changes in amino acids, catechins and alkaloids during the storage of oolong tea and their relationship with antibacterial effect.
Cui J, Wu B, Zhou J. Cui J, et al. Sci Rep. 2024 May 7;14(1):10424. doi: 10.1038/s41598-024-60951-5. Sci Rep. 2024. PMID: 38710752 Free PMC article.
References
- Ximenez-Fyvie L. A., Haffajee A. D. & Socransky S. S. Comparison of the microbiota of supra- and subgingival plaque in health and periodontitis. J Clin Periodontol 27, 648–657 (2000). - PubMed
- Kolenbrander P. E. et al.. Bacterial interactions and successions during plaque development. Periodontol 2000 42, 47–79 (2006). - PubMed
- Socransky S. S., Haffajee A. D., Cugini M. A., Smith C. & Kent R. L. Jr. Microbial complexes in subgingival plaque. J Clin Periodontol 25, 134–144 (1988). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical