TGF-β1 stimulates HDAC4 nucleus-to-cytoplasm translocation and NADPH oxidase 4-derived reactive oxygen species in normal human lung fibroblasts - PubMed (original) (raw)
TGF-β1 stimulates HDAC4 nucleus-to-cytoplasm translocation and NADPH oxidase 4-derived reactive oxygen species in normal human lung fibroblasts
Weichao Guo et al. Am J Physiol Lung Cell Mol Physiol. 2017.
Abstract
Myofibroblasts are important mediators of fibrogenesis; thus blocking fibroblast-to-myofibroblast differentiation (FMD) may be an effective strategy to treat pulmonary fibrosis (PF). Previously, we reported that histone deacetylase 4 (HDAC4) activity is necessary for transforming growth factor-β1 (TGF-β1)-induced human lung FMD. Here, we show that TGF-β1 increases NADPH oxidase 4 (NOX4) mRNA and protein expression in normal human lung fibroblasts (NHLFs) and causes nuclear export of HDAC4. Application of the NOX family inhibitor diphenyleneiodonium chloride reduces TGF-β1-induced HDAC4 nuclear export, expression of the myofibroblast marker α-smooth muscle actin (α-SMA), and α-SMA fiber formation. Inhibition of HDAC4 nucleus-to-cytoplasm translocation using leptomycin B (LMB) had little effect on α-SMA expression but blocked α-SMA fiber formation. A coimmunoprecipitation assay showed that HDAC4 associates with α-SMA. Moreover, LMB abolishes TGF-β1-induced α-SMA fiber formation and cell contraction. Relevant to human pulmonary fibrosis, idiopathic PF specimens showed significantly higher NOX4 RNA expression and scant HDAC4 staining within nuclei of fibroblast foci myofibroblasts. Taken together, these results indicate that reactive oxygen species promote TGF-β1-mediated myofibroblast differentiation and HDAC4 nuclear export. The physical association of HDAC4 with α-SMA suggests that HDAC4 has a role in regulating the α-SMA cytoskeleton arrangement.
Keywords: HDAC4; IPF; NOX4; myofibroblasts; reactive oxygen species; α-smooth muscle actin.
Copyright © 2017 the American Physiological Society.
Figures
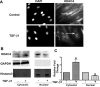
Fig. 1.
TGF-β1 promotes nuclear export of HDAC4. NHLFs were treated with TGF-β1 (1 ng/ml) for 12 h. A: immunostaining of HDAC4. Arrows indicate nuclei. DAPI, 4′,6′-diamidino-2-phenylindole. B: HDAC4 subcellular distribution in cytosolic and nuclear fractions was analyzed using immunoblot. C: densitometry measurements of triplicates for B. *P < 0.05 vs. control.
Fig. 2.
TGF-β1 stimulates NOX4-derived ROS to augment α-SMA expression in NHLFs. A: NOX4 expression was analyzed using quantitative real-time PCR 9 h after TGF-β1 treatment. B: NOX4 expression in the membrane fraction was analyzed using immunoblot. α-TU, α-tubulin. C: ROS were measured using the DCFH-DA method after TGF-β1 treatment for 12 h with or without DPI (*P < 0.05 vs. control, **P < 0.05 vs. TGF-β1 + DMSO). D: NHLFs were transfected with NOX4 siRNA or nontarget control siRNA (NC) for 48 h and then treated with TGF-β1 for 12 h. ROS were measured using the DCFH-DA method (#P < 0.05 vs. control, ##P < 0.05 vs. NC + DMSO). E: NHLFs were pretreated with DPI for 1 h followed by treatment with TGF-β1 for 12 h. α-SMA expression was assessed using immunoblot. F: immunoblot for type I human collagen 1 (h-Col1). Immunoblot results represent at least 2 independent assays. RT-PCR represents 3 independent assays.
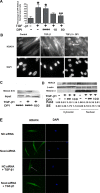
Fig. 3.
Inhibition of ROS reduces HDAC4 nuclear export. A: NHLFs were transfected with GFP-HDAC4-expressing plasmids using electroporation and then treated with TGF-β1 ± DPI for 24 h. Green fluorescent protein (GFP)-positive cells were counted for each treatment. The results represent the percentage of cells lacking nuclear GFP (*P < 0.05 vs. control, **P < 0.05 vs. TGF-β1 + DMSO). B: immunostaining of endogenous HDAC4 in NHLFs. NHLFs were treated with DPI for 2 h before TGF-β1 treatment for 12 h. C: immunoblot analysis of HDAC4 disulfide bond formation. The upper blot, “HDAC4 S=S,” represents BIAM HDAC4 (BIAM-labeled HDAC4 disulfide bonds). The lower blot, “Input,” indicates total HDAC4. D: immunoblot analysis showing HDAC4 distribution within cytoplasm and nuclear compartments. E: immunostaining of HDAC4 localization. NHLFs were pretreated with nontargeting control (NC) siRNA or NOX4 siRNA for 48 h and then treated with or without TGF-β1 for 24 h. Results represent at least 2 independent assays. Arrows indicate nuclei. DM, DMSO.
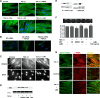
Fig. 4.
HDAC4 associates with α-SMA, and inhibition of ROS reduces myofibroblast contractility. A: immunostaining of α-SMA fibers. NHLFs were pretreated with DMSO, TSA, or LMB for 2 h and then treated with TGF-β1 for 24 h. B: immunostaining of α-SMA fibers. NHLFs were transfected with HDAC4 siRNA or nontarget control siRNA (NC) for 48 h and then treated with TGF-β1 for 24 h. C: immunostaining of endogenous HDAC4 localization. NHLFs were pretreated with LMB for 2 h and then treated with TGF-β1 for 12 h. D: immunoblot analysis of α-SMA protein expression in NHLFs treated with TGF-β1, with or without LMB or TSA. E: coimmunoprecipitation (IP) of endogenous HDAC4 with α-SMA. NHLFs were stimulated with TGF-β1 for 24 h. F: NHLF collagen gel contraction assay. The ratio of the area of gel to culture well was used to express the contractile ability (*P < 0.05 vs. control, **P < 0.05 vs. DMSO). G: colocalization of stress fibers (phalloidin staining) and α-SMA. Results represent at least 2 independent assays.
Fig. 5.
Expressions of NOX4 and HDAC4 in IPF lung biopsy specimens. A: total RNA was isolated from lung biopsy specimens of IPF patients [n = 10; 3 patients with forced vital capacity (FVC) of 50–80% predicted and 7 patients with FVC of <50% predicted] or controls [very mild chronic obstructive pulmonary disease; COPD; Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 1; n = 11]. NOX 4 mRNA expression was measured using real-time PCR and normalized to 18S. *P < 0.05 vs. control. B: immunohistochemistry localizing HDAC4 in IPF lung biopsy specimens. Scale bars are for the panoramic view 100 μm (bottom left), middle magnification 20 μm (top left), and high magnification 10 μm (top right and bottom right).
Similar articles
- Abrogation of TGF-beta1-induced fibroblast-myofibroblast differentiation by histone deacetylase inhibition.
Guo W, Shan B, Klingsberg RC, Qin X, Lasky JA. Guo W, et al. Am J Physiol Lung Cell Mol Physiol. 2009 Nov;297(5):L864-70. doi: 10.1152/ajplung.00128.2009. Epub 2009 Aug 21. Am J Physiol Lung Cell Mol Physiol. 2009. PMID: 19700647 Free PMC article. - NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts.
Bondi CD, Manickam N, Lee DY, Block K, Gorin Y, Abboud HE, Barnes JL. Bondi CD, et al. J Am Soc Nephrol. 2010 Jan;21(1):93-102. doi: 10.1681/ASN.2009020146. Epub 2009 Nov 19. J Am Soc Nephrol. 2010. PMID: 19926889 Free PMC article. - Myofibroblast differentiation during fibrosis: role of NAD(P)H oxidases.
Barnes JL, Gorin Y. Barnes JL, et al. Kidney Int. 2011 May;79(9):944-56. doi: 10.1038/ki.2010.516. Epub 2011 Feb 9. Kidney Int. 2011. PMID: 21307839 Free PMC article. Review. - Oxidative stress in cancer and fibrosis: Opportunity for therapeutic intervention with antioxidant compounds, enzymes, and nanoparticles.
Morry J, Ngamcherdtrakul W, Yantasee W. Morry J, et al. Redox Biol. 2017 Apr;11:240-253. doi: 10.1016/j.redox.2016.12.011. Epub 2016 Dec 16. Redox Biol. 2017. PMID: 28012439 Free PMC article. Review.
Cited by
- Effects of Different Concentrations of Oil Mist Particulate Matter on Pulmonary Fibrosis In Vivo and In Vitro.
Nie H, Liu H, Shi Y, Lai W, Liu X, Xi Z, Lin B. Nie H, et al. Toxics. 2022 Oct 28;10(11):647. doi: 10.3390/toxics10110647. Toxics. 2022. PMID: 36355939 Free PMC article. - HDAC8 inhibition ameliorates pulmonary fibrosis.
Saito S, Zhuang Y, Suzuki T, Ota Y, Bateman ME, Alkhatib AL, Morris GF, Lasky JA. Saito S, et al. Am J Physiol Lung Cell Mol Physiol. 2019 Jan 1;316(1):L175-L186. doi: 10.1152/ajplung.00551.2017. Epub 2018 Oct 25. Am J Physiol Lung Cell Mol Physiol. 2019. PMID: 30358439 Free PMC article. - Sirtuins and Cellular Senescence in Patients with Idiopathic Pulmonary Fibrosis and Systemic Autoimmune Disorders.
D'Agnano V, Mariniello DF, Pagliaro R, Far MS, Schiattarella A, Scialò F, Stella G, Matera MG, Cazzola M, Bianco A, Perrotta F. D'Agnano V, et al. Drugs. 2024 May;84(5):491-501. doi: 10.1007/s40265-024-02021-8. Epub 2024 Apr 17. Drugs. 2024. PMID: 38630364 Free PMC article. Review. - Specific epigenetic regulators serve as potential therapeutic targets in idiopathic pulmonary fibrosis.
Sehgal M, Jakhete SM, Manekar AG, Sasikumar S. Sehgal M, et al. Heliyon. 2022 Jun 30;8(8):e09773. doi: 10.1016/j.heliyon.2022.e09773. eCollection 2022 Aug. Heliyon. 2022. PMID: 36061031 Free PMC article. Review. - TGF‑β1: Gentlemanly orchestrator in idiopathic pulmonary fibrosis (Review).
Ye Z, Hu Y. Ye Z, et al. Int J Mol Med. 2021 Jul;48(1):132. doi: 10.3892/ijmm.2021.4965. Epub 2021 May 20. Int J Mol Med. 2021. PMID: 34013369 Free PMC article. Review.
References
- Bottomley MJ, Lo Surdo P, Di Giovine P, Cirillo A, Scarpelli R, Ferrigno F, Jones P, Neddermann P, De Francesco R, Steinkühler C, Gallinari P, Carfí A. Structural and functional analysis of the human HDAC4 catalytic domain reveals a regulatory structural zinc-binding domain. J Biol Chem 283: 26694–26704, 2008. doi:10.1074/jbc.M803514200. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 GM103629/GM/NIGMS NIH HHS/United States
- R01 HL083901/HL/NHLBI NIH HHS/United States
- U54 GM104940/GM/NIGMS NIH HHS/United States
- R01 HL083480/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous