What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically - PubMed (original) (raw)
Observational Study
. 2017 May;105(5):1086-1100.
doi: 10.3945/ajcn.116.139980. Epub 2017 Mar 29.
Courtney L Meehan 3, Mark A McGuire 4, Janet E Williams 4 5, James Foster 6, Daniel W Sellen 7, Elizabeth W Kamau-Mbuthia 8, Egidioh W Kamundia 8, Samwel Mbugua 8, Sophie E Moore 9 10, Andrew M Prentice 11, Linda J Kvist 12, Gloria E Otoo 13, Sarah L Brooker 4 5, William J Price 14, Bahman Shafii 14, Caitlyn Placek 3, Kimberly A Lackey 15, Bianca Robertson 16 17, Susana Manzano 18, Lorena Ruíz 18, Juan M Rodríguez 18, Rossina G Pareja 19, Lars Bode 20 17
Affiliations
- PMID: 28356278
- PMCID: PMC5402033
- DOI: 10.3945/ajcn.116.139980
Observational Study
What's normal? Oligosaccharide concentrations and profiles in milk produced by healthy women vary geographically
Michelle K McGuire et al. Am J Clin Nutr. 2017 May.
Abstract
Background: Human milk is a complex fluid comprised of myriad substances, with one of the most abundant substances being a group of complex carbohydrates referred to as human milk oligosaccharides (HMOs). There has been some evidence that HMO profiles differ in populations, but few studies have rigorously explored this variability.Objectives: We tested the hypothesis that HMO profiles differ in diverse populations of healthy women. Next, we examined relations between HMO and maternal anthropometric and reproductive indexes and indirectly examined whether differences were likely related to genetic or environmental variations.Design: In this cross-sectional, observational study, milk was collected from a total of 410 healthy, breastfeeding women in 11 international cohorts and analyzed for HMOs by using high-performance liquid chromatography.Results: There was an effect of the cohort (P < 0.05) on concentrations of almost all HMOs. For instance, the mean 3-fucosyllactose concentration was >4 times higher in milk collected in Sweden than in milk collected in rural Gambia (mean ± SEM: 473 ± 55 compared with 103 ± 16 nmol/mL, respectively; P < 0.05), and disialyllacto-_N_-tetraose (DSLNT) concentrations ranged from 216 ± 14 nmol/mL (in Sweden) to 870 ± 68 nmol/mL (in rural Gambia) (P < 0.05). Maternal age, time postpartum, weight, and body mass index were all correlated with several HMOs, and multiple differences in HMOs [e.g., lacto-_N_-neotetrose and DSLNT] were shown between ethnically similar (and likely genetically similar) populations who were living in different locations, which suggests that the environment may play a role in regulating the synthesis of HMOs.Conclusions: The results of this study support our hypothesis that normal HMO concentrations and profiles vary geographically, even in healthy women. Targeted genomic analyses are required to determine whether these differences are due at least in part to genetic variation. A careful examination of sociocultural, behavioral, and environmental factors is needed to determine their roles in this regard. This study was registered at clinicaltrials.gov as NCT02670278.
Keywords: breastfeeding; carbohydrates; human milk; lactation; oligosaccharides.
Figures
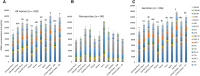
FIGURE 1
Mean ± SEM absolute total and HMO isoform concentrations of all women combined (A), nonsecretors (B), and secretors (C). (A and B) Bars without a common lowercase letter represent total HMO values that differed with the use of Bonferroni-correction procedures for multiple comparisons. All statistical inferences were carried out on log-transformed data. Note that there was only one nonsecretor subject each in Peru and United States - CA. CA, California; DFLac, difucosyllactose; DFLNH, difucosyllacto-_N_-hexaose; DFLNT, difucosyllacto-_N_-tetrose; DSLNH, disialyllacto-_N_-hexaose; DSLNT, disialyllacto-_N_-tetraose; FDSLNH, fucodisialyllacto-_N_-hexaose; FLNH, fucosyllacto-_N_-hexaose; HMO, human milk oligosaccharide; LNFP, lacto-_N_-fucopentaose; LNH, lacto-_N_-hexaose; LNnT, lacto-_N_-neotetraose; LNT, lacto-_N_-tetrose; LSTb, sialyl-lacto-_N_-tetraose b; LSTc, sialyl-lacto-_N_-tetraose c; WA, Washington; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose; 6′SL, 6′-sialyllactose.
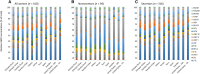
FIGURE 2
Mean ± SEM relative abundance of HMO concentrations of all women combined (A), nonsecretors (B), and secretors (C) in each cohort. Note that there was only one nonsecretor subject each in Peru and United States - CA. CA, California; DFLac, difucosyllactose; DFLNH, difucosyllacto-_N_-hexaose; DFLNT, difucosyllacto-_N_-tetrose; DSLNH, disialyllacto-_N_-hexaose; DSLNT, disialyllacto-_N_-tetraose; FDSLNH, fucodisialyllacto-_N_-hexaose; FLNH, fucosyllacto-_N_-hexaose; HMO, human milk oligosaccharide; LNFP, lacto-_N_-fucopentaose; LNH, lacto-_N_-hexaose; LNnT, lacto-_N_-neotetraose; LNT, lacto-_N_-tetrose; LSTb, sialyl-lacto-_N_-tetraose b; LSTc, sialyl-lacto-_N_-tetraose c; WA, Washington; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose; 6′SL, 6′-sialyllactose.
FIGURE 3
Percentages of women in each cohort categorized as secretors. Cohorts that do not share a common lowercase letter differ (P < 0.05) in terms of their percentages of women who were secretors with the use of a chi-square test with Benjamini and Hochberg false-discovery-rate corrections. ETR, rural Ethiopia; ETU, urban Ethiopia; GBR, rural Gambia; GBU, urban Gambia; GN, Ghana; KE, Kenya; PE, Peru; SP, Spain; SW, Sweden; USC, United States–California (Hispanic); USW, United States–Washington.
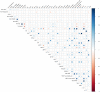
FIGURE 4
Spearman rank correlations between selected maternal anthropometric, demographic, and reproductive variables and HMO types and groupings. Sizes of dots and colors indicate directionality (blue denotes positive; red denotes negative) and the strength of the association. Total HMO-bound sialic acid; total HMO-bound fucose; small HMO; type 1; type 2; α-1,2; α-1,3; and α-2,6 were calculated as: the sum of all sialic acid moieties bound to each HMO; all fucose moieties bound to each HMO; 2′FL + 3FL + 3′SL + 6′SL; LNT + LNFPI + LNFPII + LSTb + DSLNT; LNnT + LNFPIII + LSTc; LNFP I + 2′FL; LNFP III + 3FL; and LSTb + LSTc + 6′SL, respectively. DFLac, difucosyllactose; DFLNH, difucosyllacto-_N_-hexaose; DFLNT, difucosyllacto-_N_-tetrose; DSLNH, disialyllacto-_N_-hexaose; DSLNT, disialyllacto-_N_-tetraose; FDSLNH, fucodisialyllacto-_N_-hexaose; FLNH, fucosyllacto-_N_-hexaose; HMO, human milk oligosaccharide; LNFP, lacto-_N_-fucopentaose; LNH, lacto-_N_-hexaose; LNnT, lacto-_N_-neotetraose; LNT, lacto-_N_-tetrose; LST, sialyl-lacto-_N_-tetraose; 2′FL, 2′-fucosyllactose; 3FL, 3-fucosyllactose; 3′SL, 3′-sialyllactose; 6′SL, 6′-sialyllactose.
Comment in
- Human milk oligosaccharides vary among populations.
Newburg DS. Newburg DS. Am J Clin Nutr. 2017 May;105(5):1027-1028. doi: 10.3945/ajcn.117.155721. Epub 2017 Apr 19. Am J Clin Nutr. 2017. PMID: 28424182 No abstract available.
Similar articles
- Human Milk Oligosaccharide Concentrations Are Associated with Multiple Fixed and Modifiable Maternal Characteristics, Environmental Factors, and Feeding Practices.
Azad MB, Robertson B, Atakora F, Becker AB, Subbarao P, Moraes TJ, Mandhane PJ, Turvey SE, Lefebvre DL, Sears MR, Bode L. Azad MB, et al. J Nutr. 2018 Nov 1;148(11):1733-1742. doi: 10.1093/jn/nxy175. J Nutr. 2018. PMID: 30247646 - Human Milk Oligosaccharide Profile Variation Throughout Postpartum in Healthy Women in a Brazilian Cohort.
Ferreira AL, Alves R, Figueiredo A, Alves-Santos N, Freitas-Costa N, Batalha M, Yonemitsu C, Manivong N, Furst A, Bode L, Kac G. Ferreira AL, et al. Nutrients. 2020 Mar 17;12(3):790. doi: 10.3390/nu12030790. Nutrients. 2020. PMID: 32192176 Free PMC article. Clinical Trial. - Human Milk Oligosaccharides Are Associated with Lactation Stage and Lewis Phenotype in a Chinese Population.
Ren X, Yan J, Bi Y, Shuttleworth PW, Wang Y, Jiang S, Wang J, Duan Y, Lai J, Yang Z. Ren X, et al. Nutrients. 2023 Mar 15;15(6):1408. doi: 10.3390/nu15061408. Nutrients. 2023. PMID: 36986137 Free PMC article. - The Mean of Milk: A Review of Human Milk Oligosaccharide Concentrations throughout Lactation.
Soyyılmaz B, Mikš MH, Röhrig CH, Matwiejuk M, Meszaros-Matwiejuk A, Vigsnæs LK. Soyyılmaz B, et al. Nutrients. 2021 Aug 9;13(8):2737. doi: 10.3390/nu13082737. Nutrients. 2021. PMID: 34444897 Free PMC article. Review. - Human Milk Oligosaccharides: 2'-Fucosyllactose (2'-FL) and Lacto-N-Neotetraose (LNnT) in Infant Formula.
Vandenplas Y, Berger B, Carnielli VP, Ksiazyk J, Lagström H, Sanchez Luna M, Migacheva N, Mosselmans JM, Picaud JC, Possner M, Singhal A, Wabitsch M. Vandenplas Y, et al. Nutrients. 2018 Aug 24;10(9):1161. doi: 10.3390/nu10091161. Nutrients. 2018. PMID: 30149573 Free PMC article. Review.
Cited by
- Sodium and Potassium Concentrations and Somatic Cell Count of Human Milk Produced in the First Six Weeks Postpartum and Their Suitability as Biomarkers of Clinical and Subclinical Mastitis.
Pace RM, Pace CDW, Fehrenkamp BD, Price WJ, Lewis M, Williams JE, McGuire MA, McGuire MK. Pace RM, et al. Nutrients. 2022 Nov 8;14(22):4708. doi: 10.3390/nu14224708. Nutrients. 2022. PMID: 36432395 Free PMC article. - Mothers' Breast Milk Composition and Their Respective Infant's Gut Microbiota Differ between Five Distinct Rural and Urban Regions in Vietnam.
Kortman GAM, Timmerman HM, Schaafsma A, Stoutjesdijk E, Muskiet FAJ, Nhien NV, van Hoffen E, Boekhorst J, Nauta A. Kortman GAM, et al. Nutrients. 2023 Nov 16;15(22):4802. doi: 10.3390/nu15224802. Nutrients. 2023. PMID: 38004196 Free PMC article. - The Potential Role of Human Milk Oligosaccharides in Irritable Bowel Syndrome.
Sanz Morales P, Wijeyesekera A, Robertson MD, Jackson PPJ, Gibson GR. Sanz Morales P, et al. Microorganisms. 2022 Nov 25;10(12):2338. doi: 10.3390/microorganisms10122338. Microorganisms. 2022. PMID: 36557589 Free PMC article. Review. - Health benefits conferred by the human gut microbiota during infancy.
Ventura M, Milani C, Lugli GA, van Sinderen D. Ventura M, et al. Microb Biotechnol. 2019 Mar;12(2):243-248. doi: 10.1111/1751-7915.13334. Epub 2018 Nov 8. Microb Biotechnol. 2019. PMID: 30411507 Free PMC article. - Serum and Amniotic Fluid Metabolic Profile Changes in Response to Gestational Diabetes Mellitus and the Association with Maternal-Fetal Outcomes.
Zhou Y, Zhao R, Lyu Y, Shi H, Ye W, Tan Y, Li R, Xu Y. Zhou Y, et al. Nutrients. 2021 Oct 18;13(10):3644. doi: 10.3390/nu13103644. Nutrients. 2021. PMID: 34684645 Free PMC article.
References
- Coppa GV, Pierani P, Zampini L, Carloni I, Carlucci A, Gabrielli O. Oligosaccharides in human milk during different phases of lactation. Acta Paediatr Suppl 1999;88:89–94. - PubMed
- Kunz C, Rodriguez-Palmero M, Koletzko B, Jensen R. Nutritional and biochemical properties of human milk, part I: general aspects, proteins, and carbohydrates. Clin Perinatol 1999;26:307–33. - PubMed
- Newburg DS, Shen Z, Warren CD. Quantitative analysis of human milk oligosaccharides by capillary electrophoresis. Adv Exp Med Biol 2000;478:381–2. - PubMed
- Chaturvedi P, Warren CD, Altaye M, Morrow AL, Ruiz-Palacios G, Pickering LK, Newburg DS. Fucosylated human milk oligosaccharides vary between individuals and over the course of lactation. Glycobiology 2001;11:365–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- MC_U123261345/MRC_/Medical Research Council/United Kingdom
- MC_U123292701/MRC_/Medical Research Council/United Kingdom
- MC_UP_1005/1/MRC_/Medical Research Council/United Kingdom
- MR/P012019/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources