Biocompatibility of biological material polylactic acid with stem cells from human exfoliated deciduous teeth - PubMed (original) (raw)
Biocompatibility of biological material polylactic acid with stem cells from human exfoliated deciduous teeth
Xi Wang et al. Biomed Rep. 2017 May.
Abstract
To investigate the biocompatibility of the biomaterial, polylactic acid (PLA) with stem cells from human exfoliated deciduous teeth (SHED) and its induction of mineralization as a type of scaffold material. To determine the impacts of biomaterial PLA on proliferation and mineralization of SHED, the expression of surface molecules of SHED isolated and cultured in vitro was detected by flow cytometry. In addition, cell proliferation was measured using MTT and Edu assays, and the evaluation of mineralized differentiation was performed using Alizarin Red S staining. In addition, the expression levels of osteogenic marker genes were measured by reverse transcription-quantitative polymerase chain reaction (RT-qPCR) and western blot analysis. SHED were successfully isolated and identified. The MTT and Edu results indicated that the proliferation of SHED cultured in PLA and normal medium was not significantly different. The Alizarin Red S staining demonstrated that the mineralization capability was significantly higher in the SHED that were cultured in PLA medium. Furthermore, RT-qPCR and western blot analyses indicated that the expression levels of osteogenic marker genes were higher in the SHED cultured in PLA medium. These results suggested that PLA possesses good biocompatibility with SHED and may effectively induce the mineralization of SHED and serve as a scaffold material.
Keywords: biocompatibility; osteogenic induction; polylactic acid; stem cells from human exfoliated deciduous teeth.
Figures
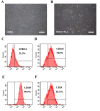
Figure 1.
Cell morphology and FCM analysis of SHED. (A) Cell morphology of SHED incubated with α-MEM containing 5% FBS. (B) Cell morphology of SHED incubated with 100% PLA extract. (C-F) Representative images of FCM data for SHED. Scale bars, 50 µm. FCM, flow cytometric; SHED, stem cells from human exfoliated deciduous teeth; PLA, polylactic acid; α-MEM, α-minimum essential medium; CD146, cluster of differentiation 146.
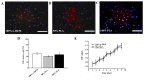
Figure 2.
Proliferation ability of SHED cultured in normal medium and media with PLA extract. (A-C) Representative EdU staining of SHED cultured in normal medium and media with varying quantities of PLA extract. Cells with red nuclei are proliferating cells. (D) Percentage of EdU-positive cells (number of red nuclei). (E) Growth curves for SHED cultured in different media were analyzed using the MTT assay. The experimental results demonstrated that the number of proliferating cells was not significantly different between the three groups (P>0.1). The data shown represent mean ± standard deviation. Scale bars, 50 µm. SHED, stem cells from human exfoliated deciduous teeth; PLA, polylactic acid; α-MEM, α-minimum essential medium; OD, optical density.
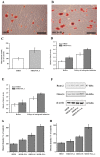
Figure 3.
Osteogenic differentiation ability of SHED cultured in normal medium and medium with PLA extract. (A and B) Mineralization nodules formed in SHED cultured in normal medium and medium with PLA extract. Scale bars, 50 µm. (C) The graph presents the statistically significant difference in the number of mineralization nodules between the groups. (D and E) The osteogenesis-associated gene expression profiles of SHED cultured in various media were detected using reverse transcription-quantitative polymerase chain reaction. The results indicated that following osteogenic induction, the expression levels of RUNX2 and osterix were increased in all treatment groups; however, these increases were significantly higher in SHED cultured in media containing PLA extract. The data are presented as means ± standard deviation. (F) The expression of RUNX2 and osterix of SHED cultured in various media were analyzed by western blotting. Western blot analysis indicated that the expression levels of these proteins were increased following osteogenic induction; however, these increases were significantly higher in cells cultured in medium containing PLA extract. (G and H) Quantification of these data. *P<0.05 vs. SHED. SHED, stem cells from human exfoliated deciduous teeth; PLA, polylactic acid; RUNX2, runt-related transcription factor 2; os, osteogenesis.
Similar articles
- [Comparison of the properties of CD146 positive and CD146 negative subpopulations of stem cells from human exfoliated deciduous teeth].
Wang XT, Rao NQ, Fang TJ, Zhao YM, Ge LH. Wang XT, et al. Beijing Da Xue Xue Bao Yi Xue Ban. 2018 Apr 18;50(2):284-292. Beijing Da Xue Xue Bao Yi Xue Ban. 2018. PMID: 29643528 Chinese. - [Difference of in vitro osteogenic differentiation and osteoclast capacity between stem cells from human exfoliated deciduous teeth and dental pulp stem cells].
Lu BW, Liu N, Xu LL, Shi HG, Zhang Y, Zhang W. Lu BW, et al. Nan Fang Yi Ke Da Xue Xue Bao. 2016 Feb;36(2):180-5. Nan Fang Yi Ke Da Xue Xue Bao. 2016. PMID: 26922012 Chinese. - Interleukin-17A promotes osteogenic differentiation by increasing OPG/RANKL ratio in stem cells from human exfoliated deciduous teeth (SHED).
Sebastian AA, Kannan TP, Norazmi MN, Nurul AA. Sebastian AA, et al. J Tissue Eng Regen Med. 2018 Aug;12(8):1856-1866. doi: 10.1002/term.2706. Epub 2018 Jun 25. J Tissue Eng Regen Med. 2018. PMID: 29774992 - In vitro and in vivo characteristics of stem cells from human exfoliated deciduous teeth obtained by enzymatic disaggregation and outgrowth.
Jeon M, Song JS, Choi BJ, Choi HJ, Shin DM, Jung HS, Kim SO. Jeon M, et al. Arch Oral Biol. 2014 Oct;59(10):1013-23. doi: 10.1016/j.archoralbio.2014.06.002. Epub 2014 Jun 11. Arch Oral Biol. 2014. PMID: 24960116 - Stem Cells from Human Exfoliated Deciduous Teeth: A Growing Literature.
Martinez Saez D, Sasaki RT, Neves AD, da Silva MC. Martinez Saez D, et al. Cells Tissues Organs. 2016;202(5-6):269-280. doi: 10.1159/000447055. Epub 2016 Aug 20. Cells Tissues Organs. 2016. PMID: 27544531 Review.
Cited by
- Osteoblastic Differentiation of Stem Cells from Human Exfoliated Deciduous Teeth by Probiotic Hydroxyapatite.
Nouri S, Roghanian R, Emtiazi G, Gunduz O, Shafiei R. Nouri S, et al. Cell J. 2023 Nov 28;25(11):753-763. doi: 10.22074/cellj.2023.1999743.1276. Cell J. 2023. PMID: 38071407 Free PMC article. - Scaffolds for Dentin-Pulp Complex Regeneration.
Sequeira DB, Diogo P, Gomes BPFA, Peça J, Santos JMM. Sequeira DB, et al. Medicina (Kaunas). 2023 Dec 20;60(1):7. doi: 10.3390/medicina60010007. Medicina (Kaunas). 2023. PMID: 38276040 Free PMC article. Review. - Accelerated degradation testing impacts the degradation processes in 3D printed amorphous PLLA.
Malone LP, Best SM, Cameron RE. Malone LP, et al. Front Bioeng Biotechnol. 2024 Jul 5;12:1419654. doi: 10.3389/fbioe.2024.1419654. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39036561 Free PMC article. - Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration.
Diomede F, Gugliandolo A, Scionti D, Merciaro I, Cavalcanti MF, Mazzon E, Trubiani O. Diomede F, et al. Int J Mol Sci. 2018 Jan 23;19(2):329. doi: 10.3390/ijms19020329. Int J Mol Sci. 2018. PMID: 29360771 Free PMC article. - Characterization of Biological Properties of Dental Pulp Stem Cells Grown on an Electrospun Poly(l-lactide-_co_-caprolactone) Scaffold.
Bar JK, Kowalczyk T, Grelewski PG, Stamnitz S, Paprocka M, Lis J, Lis-Nawara A, An S, Klimczak A. Bar JK, et al. Materials (Basel). 2022 Mar 3;15(5):1900. doi: 10.3390/ma15051900. Materials (Basel). 2022. PMID: 35269131 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources