Vaginal Lactobacillus Inhibits HIV-1 Replication in Human Tissues Ex Vivo - PubMed (original) (raw)
Vaginal Lactobacillus Inhibits HIV-1 Replication in Human Tissues Ex Vivo
Rogers A Ñahui Palomino et al. Front Microbiol. 2017.
Abstract
Lactobacillus species, which dominate vaginal microbiota of healthy reproductive-age women, lower the risks of sexually transmitted infections, including the risk of human immunodeficiency virus (HIV) acquisition. The exact mechanisms of this protection remain to be understood. Here, we investigated these mechanisms in the context of human cervico-vaginal and lymphoid tissues ex vivo. We found that all six Lactobacillus strains tested in these systems significantly suppressed HIV type-1 (HIV-1) infection. We identified at least three factors that mediated this suppression: (i) Acidification of the medium. The pH of the undiluted medium conditioned by lactobacilli was between 3.8 and 4.6. Acidification of the culture medium with hydrochloric acid (HCl) to this pH in control experiments was sufficient to abrogate HIV-1 replication. However, the pH of the _Lactobacillus_-conditioned medium (CM) diluted fivefold, which reached ∼6.9, was also suppressive for HIV-1 infection, while in control experiments HIV-1 infection was not abrogated when the pH of the medium was brought to 6.9 through the use of HCl. This suggested the existence of other factors responsible for HIV-1 inhibition by lactobacilli. (ii) Lactic acid. There was a correlation between the concentration of lactic acid in the _Lactobacillus_-CM and its ability to suppress HIV-1 infection in human tissues ex vivo. Addition of lactic acid isomers D and L to tissue culture medium at the concentration that corresponded to their amount released by lactobacilli resulted in HIV-1 inhibition. Isomer L was produced in higher quantities than isomer D and was mostly responsible for HIV-1 inhibition. These results indicate that lactic acid, in particular its L-isomer, inhibits HIV-1 independently of lowering of the pH. (iii) Virucidal effect. Incubation of HIV-1 in _Lactobacillus_-CM significantly suppressed viral infectivity for human tissues ex vivo. Finally, lactobacilli adsorb HIV-1, serving as a sink decreasing the number of free virions. In summary, we found that lactobacilli inhibit HIV-1 replication in human tissue ex vivo by multiple mechanisms. Further studies are needed to evaluate the potential of altering the spectra of vaginal microbiota as an effective strategy to enhance vaginal health. Human tissues ex vivo may serve as a test system for these strategies.
Keywords: HIV-1; Lactobacillus; human tissue; lactic acid; pH.
Figures
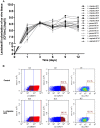
FIGURE 1
Lactobacillus colonization of ex vivo tissue. (A) Tonsillar tissues were colonized with 15 vaginal Lactobacillus strains (L. crispatus BC1, BC3–BC8; L. gasseri BC9–BC14 and L. vaginalis BC16, BC17), at a starting inoculum of 104 CFU/mL and cultured for 12 days. We evaluated Lactobacillus colonization every 3 days by measuring OD600 using a spectrophotometer. Bars represent mean ± SD from tissues of three donors. (B) We evaluated tissue cell depletion induced by Lactobacillus colonization of ex vivo tissues 3 days after bacterial inoculation using flow cytometry. Panels (from left to right) represent live/dead staining, CD3+ expression in live cells, and Bcl2 expression in CD3+ cells in control (upper row) and L. crispatus BC5-colonized tissue (lower row).
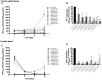
FIGURE 2
HIV-1 infection of human tissue ex vivo treated with _Lactobacillus_-CM. Cervico-vaginal (A,B) and tonsillar (C,D) tissue blocks were pre-incubated with undiluted and diluted 1:5 Lactobacillus_-CM from six strains (L. crispatus BC3 and BC5; L. gasseri BC12 and BC13; and L. vaginalis BC16 and BC17). Tissue cultures were inoculated with HIV-1 and kept in the Lactobacillus_-CM for 3 days. At day 3, the Lactobacillus_-CM was removed and cultures were kept in regular medium until day 12 post-inoculation. (A,C) We evaluated the kinetics of HIV-1 replication in tissues by measuring the levels of p24gag in tissue culture medium. (B,D) Replication of HIV-1 in Lactobacillus_-treated tissues expressed as percentages of HIV-1 replication in untreated control (black bars). Statistical significance vs. control is presented. Bars represent mean ± SD from five tissue donors. Asterisks indicate statistical significance by one-way ANOVA multiple comparison (∗_p < 0.05, ∗∗_p < 0.01, ∗∗∗_p < 0.001, ∗∗∗∗_p < 0.0001). 3TC (dideoxythiacytidine or lamivudine) at 10 μM is a powerful HIV-1 inhibitor that we used in our study as a positive control.
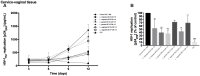
FIGURE 3
Virucidal effect of _Lactobacillus_-CM against HIV-1. Virucidal capacities of six strains, L. crispatus (BC3 and BC5); L. gasseri (BC12 and BC13); and L. vaginalis (BC16, BC17) are presented. HIV-1 preparation was pretreated with Lactobacillus_-CM diluted 1:5 for 1 h, and HIV-1 infectivity was tested in cervico-vaginal tissues ex vivo. (A) We evaluated the kinetics of HIV-1 replication by measuring the levels of p24gag in tissue culture medium. (B) Replication of HIV-1 in Lactobacillus_-treated tissues expressed as percentages of HIV-1 replication in untreated control (black bars). Statistical significance vs. control is presented. Bars represent mean ± SD from tissues of five patients. Asterisks indicate statistical significance by one-way ANOVA multiple comparison (∗_p < 0.05, ∗∗_p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). 3TC (dideoxythiacytidine or lamivudine) at 10 μM is a powerful HIV-1 inhibitor that we used in our study as a positive control.
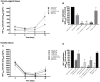
FIGURE 4
Effect of lactic acid isomers D and L and of pH on HIV-1 replication. The effects of lactate isomers D and L at concentrations found in Lactobacillus_-CM on HIV-1 replication were tested in cervico-vaginal (A,B) and tonsillar (C,D) tissues. Isomers D and L were respectively tested at 3 and 23 mM concentrations, which correspond to the average concentrations of lactic acid found in Lactobacillus_-CM. The mixture of isomers L and D was tested at the concentrations of 3 and 23 mM. We evaluated the effect of acidic pH on HIV-1 infectivity in ex vivo tissues by buffering the culture medium at pH 4 and pH 6.9 using HCl. (A,C) We evaluated the kinetics of HIV-1 replication in tissues by measuring the levels of p24gag in culture medium. (B,D) Replication of HIV-1 in Lactobacillus_-treated tissues was expressed as percentage of HIV-1 replication in untreated control (black bars). Statistical significance vs. control is presented. Bars represent mean ± SD from tissues of three to five donors. Asterisks indicate statistical significance by one-way ANOVA multiple comparison (∗_p < 0.05, ∗∗_p < 0.01, ∗∗∗_p < 0.001, ∗∗∗∗p < 0.0001).
FIGURE 5
Virucidal effect of Lactobacillus against HIV-1. Virucidal capacity of six strains, L. crispatus (BC3, BC5), L. gasseri (BC12, BC13), and L. vaginalis (BC16, BC17) is presented. HIV-1 was pre-treated with Lactobacillus_-CP at 108 CFU/mL, and HIV-1 infectivity was then tested in cervico-vaginal tissues ex vivo. (A) We evaluated HIV-1 replication kinetics in tissues by measuring p24gag in culture medium. (B) Replication of HIV-1 in Lactobacillus_-treated tissues was expressed as percentage of HIV-1 replication in untreated control (black bars). (C) Fractions of p24gag associated with CP after incubation with HIV-1 are presented. Statistical significance vs. control was calculated. Bars represent mean ± SD from five patients. Asterisks indicate statistical significance by one-way ANOVA multiple comparison (∗_p < 0.05, ∗∗_p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001). 3TC (dideoxythiacytidine or lamivudine) at 10 μM is a powerful HIV-1 inhibitor that we used in our study as a positive control.
Similar articles
- Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid.
Tyssen D, Wang YY, Hayward JA, Agius PA, DeLong K, Aldunate M, Ravel J, Moench TR, Cone RA, Tachedjian G. Tyssen D, et al. mSphere. 2018 Jul 5;3(4):e00055-18. doi: 10.1128/mSphere.00055-18. mSphere. 2018. PMID: 29976641 Free PMC article. - Vaginal pH measured in vivo: lactobacilli determine pH and lactic acid concentration.
O'Hanlon DE, Come RA, Moench TR. O'Hanlon DE, et al. BMC Microbiol. 2019 Jan 14;19(1):13. doi: 10.1186/s12866-019-1388-8. BMC Microbiol. 2019. PMID: 30642259 Free PMC article. - Vaginal concentrations of lactic acid potently inactivate HIV.
Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, Cone R, Tachedjian G. Aldunate M, et al. J Antimicrob Chemother. 2013 Sep;68(9):2015-25. doi: 10.1093/jac/dkt156. Epub 2013 May 8. J Antimicrob Chemother. 2013. PMID: 23657804 Free PMC article. - Vaginal microbiome.
Buchta V. Buchta V. Ceska Gynekol. 2018 Winter;83(5):371-379. Ceska Gynekol. 2018. PMID: 30848142 Review. English. - Why do lactobacilli dominate the human vaginal microbiota?
Witkin SS, Linhares IM. Witkin SS, et al. BJOG. 2017 Mar;124(4):606-611. doi: 10.1111/1471-0528.14390. Epub 2016 Nov 7. BJOG. 2017. PMID: 28224747 Review.
Cited by
- Lactic acid from vaginal microbiota enhances cervicovaginal epithelial barrier integrity by promoting tight junction protein expression.
Delgado-Diaz DJ, Jesaveluk B, Hayward JA, Tyssen D, Alisoltani A, Potgieter M, Bell L, Ross E, Iranzadeh A, Allali I, Dabee S, Barnabas S, Gamieldien H, Blackburn JM, Mulder N, Smith SB, Edwards VL, Burgener AD, Bekker LG, Ravel J, Passmore JS, Masson L, Hearps AC, Tachedjian G. Delgado-Diaz DJ, et al. Microbiome. 2022 Aug 31;10(1):141. doi: 10.1186/s40168-022-01337-5. Microbiome. 2022. PMID: 36045402 Free PMC article. - Differential effects of depot medroxyprogesterone acetate administration on vaginal microbiome in Hispanic White and Black women.
Yang L, Hao Y, Hu J, Kelly D, Li H, Brown S, Tasker C, Roche NE, Chang TL, Pei Z. Yang L, et al. Emerg Microbes Infect. 2019;8(1):197-210. doi: 10.1080/22221751.2018.1563458. Emerg Microbes Infect. 2019. PMID: 30866773 Free PMC article. - Metabolites with SARS-CoV-2 Inhibitory Activity Identified from Human Microbiome Commensals.
Piscotta FJ, Hoffmann HH, Choi YJ, Small GI, Ashbrook AW, Koirala B, Campbell EA, Darst SA, Rice CM, Brady SF. Piscotta FJ, et al. mSphere. 2021 Dec 22;6(6):e0071121. doi: 10.1128/mSphere.00711-21. Epub 2021 Dec 1. mSphere. 2021. PMID: 34851166 Free PMC article. - The Vaginal Microbiome of Nonhuman Primates Can Be Only Transiently Altered to Become Lactobacillus Dominant without Reducing Inflammation.
Langner CA, Ortiz AM, Flynn JK, Kendall H, Lagenaur LA, Brenchley JM. Langner CA, et al. Microbiol Spectr. 2021 Dec 22;9(3):e0107421. doi: 10.1128/Spectrum.01074-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756073 Free PMC article. - Anti-HIV-1 Activity of Lactic Acid in Human Cervicovaginal Fluid.
Tyssen D, Wang YY, Hayward JA, Agius PA, DeLong K, Aldunate M, Ravel J, Moench TR, Cone RA, Tachedjian G. Tyssen D, et al. mSphere. 2018 Jul 5;3(4):e00055-18. doi: 10.1128/mSphere.00055-18. mSphere. 2018. PMID: 29976641 Free PMC article.
References
- Aleshkin V. A., Voropaeva E. A., Shenderov B. A. (2011). Vaginal microbiota in healthy women and patients with bacterial vaginosis and nonspecific vaginitis. Microb. Ecol. Health Dis. 18 71–74. 10.3402/mehd.v18i2.7694 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources