Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects - PubMed (original) (raw)
Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects
Francesca Accattato et al. PLoS One. 2017.
Abstract
Circulating oxidative stress and pro-inflammatory markers change after regular physical exercise; however, how a short session of acute physical activity affects the inflammatory status and redox balance in sedentary individuals is still unclear. Aim of this study is to assess antioxidant and inflammatory parameters, both at rest and after acute exercise, in sedentary young men with or without obesity. Thirty sedentary male volunteers, aged 20-45 (mean age 32 ± 7 years), were recruited, divided into 3 groups (normal weight: BMI < 25 kg/m2; overweight to moderate obesity: 25-35 kg/m2; severe obesity: 35-40 kg/m2), and their blood samples collected before and after a 20-min run at ~ 70% of their VO2max for the measurement of Glutathione Reductase, Glutathione Peroxidase, Superoxide Dismutase, Total Antioxidant Status (TAS) and cytokines (IL-2, IL-4, IL-6, IL-8, IL-10, IL-1α, IL-1β, TNFα, MCP-1, VEGF, IFNγ, EGF). Inter-group comparisons demonstrated significantly higher Glutathione Reductase activity in severely obese subjects in the post-exercise period (P = 0.036), and higher EGF levels in normal weight individuals, either before (P = 0.003) and after exercise (P = 0.05). Intra-group comparisons showed that the acute exercise stress induced a significant increase in Glutathione Reductase activity in severely obese subjects only (P = 0.007), a significant decrease in MCP-1 in the normal weight group (P = 0.02), and a decrease in EGF levels in all groups (normal weight: P = 0.025, overweight/moderate obesity: P = 0.04, severe obesity: P = 0.018). Altogether, these findings suggest that in sedentary individuals with different ranges of BMI, Glutathione Reductase and distinct cytokines are differentially involved into the adaptive metabolic changes and redox responses induced by physical exercise. Therefore, these biomarkers may have the potential to identify individuals at higher risk for developing diseases pathophysiologically linked to oxidative stress.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
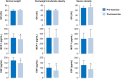
Fig 1. Pre- and post-exercise circulating GR, MCP-1, and EGF in the different BMI categories.
Analytes were determined before and after 20 min exercise session at ~70% VO2max in normal weight (BMI: <25 kg/m2, n = 10), overweight/moderate obese (BMI: 25–35 kg/m2, n = 10), severe obese (BMI: 35–40 kg/m2, n = 10) subjects. Data are expressed as mean ± SD.* denotes pre- vs. post-exercise statistical difference (P < 0.05).
Similar articles
- Antioxidant status and oxidative stress at rest and in response to acute exercise in judokas and sedentary men.
El Abed K, Rebai H, Bloomer RJ, Trabelsi K, Masmoudi L, Zbidi A, Sahnoun Z, Hakim A, Tabka Z. El Abed K, et al. J Strength Cond Res. 2011 Sep;25(9):2400-9. doi: 10.1519/JSC.0b013e3181fc5c35. J Strength Cond Res. 2011. PMID: 21869626 - Cytokine profiles in overweight and obese subjects and normal weight individuals matched for age and gender.
Azizian M, Mahdipour E, Mirhafez SR, Shoeibi S, Nematy M, Esmaily H, Ferns GA, Ghayour-Mobarhan M. Azizian M, et al. Ann Clin Biochem. 2016 Nov;53(6):663-668. doi: 10.1177/0004563216629997. Epub 2016 Sep 28. Ann Clin Biochem. 2016. PMID: 26787627 - Blood MCP-1 levels are increased in chronic obstructive pulmonary disease patients with prevalent emphysema.
Di Stefano A, Coccini T, Roda E, Signorini C, Balbi B, Brunetti G, Ceriana P. Di Stefano A, et al. Int J Chron Obstruct Pulmon Dis. 2018 May 24;13:1691-1700. doi: 10.2147/COPD.S159915. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29872287 Free PMC article. - Oxidative Stress Modulation Through Habitual Physical Activity.
Boccatonda A, Tripaldi R, Davì G, Santilli F. Boccatonda A, et al. Curr Pharm Des. 2016;22(24):3648-80. doi: 10.2174/1381612822666160413123806. Curr Pharm Des. 2016. PMID: 27072166 Review. - Exercise tolls the bell for key mediators of low-grade inflammation in dysmetabolic conditions.
Della Guardia L, Codella R. Della Guardia L, et al. Cytokine Growth Factor Rev. 2021 Dec;62:83-93. doi: 10.1016/j.cytogfr.2021.09.003. Epub 2021 Sep 21. Cytokine Growth Factor Rev. 2021. PMID: 34620559 Review.
Cited by
- Laboratory Parameters of Hemostasis, Adhesion Molecules, and Inflammation in Type 2 Diabetes Mellitus: Correlation with Glycemic Control.
Palella E, Cimino R, Pullano SA, Fiorillo AS, Gulletta E, Brunetti A, Foti DP, Greco M. Palella E, et al. Int J Environ Res Public Health. 2020 Jan 1;17(1):300. doi: 10.3390/ijerph17010300. Int J Environ Res Public Health. 2020. PMID: 31906326 Free PMC article. - Lupinus angustifolius Protein Hydrolysates Reduce Abdominal Adiposity and Ameliorate Metabolic Associated Fatty Liver Disease (MAFLD) in Western Diet Fed-ApoE-/- Mice.
Santos-Sánchez G, Cruz-Chamorro I, Álvarez-Ríos AI, Fernández-Santos JM, Vázquez-Román MV, Rodríguez-Ortiz B, Álvarez-Sánchez N, Álvarez-López AI, Millán-Linares MDC, Millán F, Pedroche J, Fernández-Pachón MS, Lardone PJ, Guerrero JM, Bejarano I, Carrillo-Vico A. Santos-Sánchez G, et al. Antioxidants (Basel). 2021 Jul 29;10(8):1222. doi: 10.3390/antiox10081222. Antioxidants (Basel). 2021. PMID: 34439470 Free PMC article. - The Role of Antioxidants Supplementation in Clinical Practice: Focus on Cardiovascular Risk Factors.
Cammisotto V, Nocella C, Bartimoccia S, Sanguigni V, Francomano D, Sciarretta S, Pastori D, Peruzzi M, Cavarretta E, D'Amico A, Castellani V, Frati G, Carnevale R, Group S. Cammisotto V, et al. Antioxidants (Basel). 2021 Jan 20;10(2):146. doi: 10.3390/antiox10020146. Antioxidants (Basel). 2021. PMID: 33498338 Free PMC article. Review. - Effect of short duration moderate intensity physical activity on glycemic control and antioxidant status of prediabetic population.
Shah Z, Shah I, Malik MO, Ullah I. Shah Z, et al. Saudi Med J. 2021 Jun;42(6):660-665. doi: 10.15537/smj.2021.42.6.20210019. Saudi Med J. 2021. PMID: 34078729 Free PMC article. - The Effect of Moderate-Intensity Physical Exercise on Some Serum Inflammation Markers and the Immune System in Rats Fed Intermittent Fasting with a High-Fat Diet.
Günbatar N, Bulduk B, Bezgin S, Oto G, Bayıroğlu F, Bulduk M. Günbatar N, et al. Medicina (Kaunas). 2023 Sep 20;59(9):1687. doi: 10.3390/medicina59091687. Medicina (Kaunas). 2023. PMID: 37763806 Free PMC article.
References
- Barbieri E, Sestili P. Reactive oxygen species in skeletal muscle signaling. J Signal Transduct. 2012: 982794 doi: 10.1155/2012/982794 - DOI - PMC - PubMed
- Sakellariou GK, Jackson MJ, Vasilaki A. Redefining the major contributors to superoxide production in contracting skeletal muscle. The role of NAD(P)H oxidases. Free Radic Res. 2014; 48: 12–29. doi: 10.3109/10715762.2013.830718 - DOI - PubMed
- Powers SK, Ji LL, Kavazis AN, Jackson MJ. Reactive oxygen species: impact on skeletal muscle. Compr Physiol. 2011; 1: 941–969. doi: 10.1002/cphy.c100054 - DOI - PMC - PubMed
- Ghiselli A, Serafini M, Natella F, Scaccini C. Total antioxidant capacity as a tool to assess redox status: critical view and experimental data. Free Radic Biol Med. 2000; 29(11): 1106–1114. - PubMed
- Prior RL. Plasma antioxidant measurements. J Nutr. 2004; 134(11): 3184S–3185S. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous