Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes - PubMed (original) (raw)
Plasma Extracellular Vesicles Enriched for Neuronal Origin: A Potential Window into Brain Pathologic Processes
Maja Mustapic et al. Front Neurosci. 2017.
Abstract
Our team has been a pioneer in harvesting extracellular vesicles (EVs) enriched for neuronal origin from peripheral blood and using them as a biomarker discovery platform for neurological disorders. This methodology has demonstrated excellent diagnostic and predictive performance for Alzheimer's and other neurodegenerative diseases in multiple studies, providing a strong proof of concept for this approach. Here, we describe our methodology in detail and offer further evidence that isolated EVs are enriched for neuronal origin. In addition, we present evidence that EVs enriched for neuronal origin represent a more sensitive and accurate base for biomarkers than plasma, serum, or non-enriched total plasma EVs. Finally, we proceed to investigate the protein content of EVs enriched for neuronal origin and compare it with other relevant enriched and non-enriched populations of plasma EVs. Neuronal-origin enriched plasma EVs contain higher levels of signaling molecules of great interest for cellular metabolism, survival, and repair, which may be useful as biomarkers and to follow response to therapeutic interventions in a mechanism-specific manner.
Keywords: Alzheimer's disease; biological markers; extracellular vesicles (EVs); liquid biopsy diagnostics; phosphorylated tau protein.
Figures
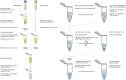
Figure 1
Graphical workflow of the neuronal enrichment protocol. W/i signifies the addition of protease and phosphatase inhibitors containing 2–3x the concentration recommended by the manufacturer.
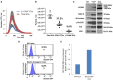
Figure 2
Neuronal EVs are found in the circulation. (A) Total EVs and L1CAM+ EVs were isolated from plasma of nine healthy volunteers and their size distribution was examined by NTA using Nanosight-NS500. Figure depicts concentration after 1:1,000 dilution for Total EVs and 1:200 dilution for L1CAM+ EVs; actual concentrations are depicted in (B). (B) The graph shows the actual concentration of total EVs and L1CAM+ and CD81+ plasma EVs immunoprecipitated from plasma of nine healthy volunteers after adjusting for dilution. The percentages in the graph represent the ratio over total EVs. (C) Western blot image shows enrichment of neuronal markers (L1CAM, MAP-2, N-enolase, p-Tau235, and TUJ) in L1CAM+ EVs when compared to total EVs from a single healthy control. CD9 is a common exosomal marker present in EVs but not in the mouse brain lysate used as a positive control. An equivalent amount of EVs was loaded on the gel by adjusting the dilution of the isolates according to the EV concentration determined by NTA. (D) GFP levels evaluated by FACS; L1CAM+ EVs were isolated from 300 μl plasma derived from Nestin-GFP transgenic or WT mice. The EVs were conjugated with the beads and the levels of GFP were evaluated by FACS analysis. The results show the percentages of beads-antibody-EV complex that contained GFP above the detection threshold. (E) GFP levels evaluated by fluorescence; comparison between the levels of GFP in EVs in the samples described in (D) were measured by plate reader at excitation of 485 nm and emission 515 nm.
Figure 3
Immuno Electron Microscopy images of L1CAM+ and CD81+ EVs. L1CAM+ or CD81+EVs were incubated with primary human anti-L1CAM or CD81 antibody followed by a secondary antibody conjugated with 6 nm gold particle. Scale bar = 100 nm, microscope settings; 120 kV, magnification 200,000x except the L1CAM+ labeled with anti-L1CAM (top left) which used a magnification of 160000x.
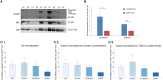
Figure 4
L1CAM+ EVs are enriched for neuronal origin. (A) L1CAM+ and CD81+ plasma-derived EVs from five healthy volunteers (out of 10 showing similar data). Western blots of L1CAM+ EVs (L1+) are set adjacent to corresponding CD81+ EVs (81+) for each individual. The membrane was stained for L1CAM (top), neuron-specific enolase (NSE; middle), and EV marker CD9 (bottom). An equivalent amount of EVs were loaded on the gel by adjusting the dilution of the isolates according to the EV concentration determined by NTA. (B) Enrichment of neuronal markers in L1CAM+ EVs compared to CD81+ EVs by Western blots [L1CAM+ EVs (Blue, N = 10); CD81+ EVs (Red, N = 10)]. Enrichment is expressed as a fold difference in the ratio of L1CAM or NSE over CD9 signal. ImageJ was used to determine the signal intensity of each marker. A paired _t_-test was used to determine statistical differences between L1CAM+ and CD81+ EVs, error bars represent SEM of 10 subjects. Significance *p < 0.05, **p < 0.0001. (C) Enrichment of neuronal markers in L1CAM+ EVs compared to CD81+ EVs by ELISA for neuronal markers, NFL, NCAM, BDNF, proBDNF. (1) Fold difference in protein levels in L1CAM+ EVs to CD81+ EVs: L1CAM+ EVs contain 2.44 ± 0.56 (mean ± SEM) fold more NFL, 2.85 ± 1.19-fold more NCAM, and 2.16 ± 0.49-fold more proBDNF than CD81+ EVs (N = 10 healthy volunteers, measured in duplicate). L1CAM+ EVs contain amounts (0.94 ± 0.05) of BDNF similar to those of CD81+ EVs. (2) Fold difference in protein levels in L1CAM+ EVs to CD81+ EVs normalized to number of EV particles/ml measured by NTA. (3) Fold difference in protein levels in L1CAM+ EVs to CD81+ EVs normalized to TSG101 protein levels measured using custom electroluminescence assay. These results show that L1CAM+ EVs contain consistently and substantially higher levels of a range of neuronal proteins compared to total and control sub-populations.
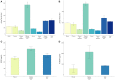
Figure 5
L1CAM+ EVs offer a higher detection level for p-tau, BDNF and pro-BDNF over plasma, serum and total EVs. For p-tau comparisons, total EVs were isolated from four plasma and serum samples from healthy volunteers followed by L1CAM immunoprecipitation. The levels of p-tau-Thr181 (A) and p-tau-Thr231 (B) are presented in the graph in L1CAM+ EVs, total EVs, plasma, serum, and in comparison, to the background signal (blank). Column bars represent the mean of four samples, error bars represent SEM. For BDNF and proBDNF comparisons, total EVs were isolated from 20 plasma samples from healthy volunteers followed by L1CAM immunoprecipitation. BDNF levels (C) are different depending on the type of fluid tested [F(2, 57) = 6.868, p = 0.002]; its levels are higher in L1CAM + EVs compared to plasma (p = 0.001) and total EVs (p = 0.016), whereas its levels in total EVs were no different than plasma (p = 0.254). Similarly, proBDNF levels (D) are dependent on the type of fluid tested [F(2, 57) = 4.41, p = 0.017]; its levels are higher in L1CAM + EVs compared to plasma (p = 0.007) and total EVs (p = 0.026), whereas its levels in total EVs were no different than plasma (p = 0.628).
Figure 6
L1CAM+ EVs contain a distinct and interesting protein signature. Total EVs were isolated from plasma from four healthy volunteers and subsequently immunoprecipitated with L1CAM or EpCAM antibodies. The samples from the four subjects were then pooled to generate a sufficient amount of protein for five membrane antibody arrays [Human Obesity Antibody Array (A), Human Phospho-Kinase Antibody Array (B), MAPK Antibody Array (C), Human Kidney Biomarker Antibody Array (D), and Human Apoptosis Antibody Array **(E)**]. An equivalent amount of protein per sample type was loaded to each spot. The levels of different proteins were calculated by densitometric analysis and normalized to the reference proteins present on each membrane. Results are depicted as heatmaps ranging from zero to maximum value and scaled according to the color bars.
Similar articles
- Assessment of technical and clinical utility of a bead-based flow cytometry platform for multiparametric phenotyping of CNS-derived extracellular vesicles.
Brahmer A, Geiß C, Lygeraki A, Neuberger E, Tzaridis T, Nguyen TT, Luessi F, Régnier-Vigouroux A, Hartmann G, Simon P, Endres K, Bittner S, Reiners KS, Krämer-Albers EM. Brahmer A, et al. Cell Commun Signal. 2023 Oct 6;21(1):276. doi: 10.1186/s12964-023-01308-9. Cell Commun Signal. 2023. PMID: 37803478 Free PMC article. - Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.
Pulliam L, Sun B, Mustapic M, Chawla S, Kapogiannis D. Pulliam L, et al. J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review. - Extracellular vesicles in the study of Alzheimer's and Parkinson's diseases: Methodologies applied from cells to biofluids.
Vaz M, Soares Martins T, Henriques AG. Vaz M, et al. J Neurochem. 2022 Nov;163(4):266-309. doi: 10.1111/jnc.15697. Epub 2022 Oct 22. J Neurochem. 2022. PMID: 36156258 Free PMC article. Review. - Exosome Biomarkers Revolutionize Preclinical Diagnosis of Neurodegenerative Diseases and Assessment of Treatment Responses in Clinical Trials.
Kapogiannis D. Kapogiannis D. Adv Exp Med Biol. 2020;1195:149. doi: 10.1007/978-3-030-32633-3_19. Adv Exp Med Biol. 2020. PMID: 32468469 Review. - Biomarker and therapeutic potential of peripheral extracellular vesicles in Alzheimer's disease.
Vandendriessche C, Kapogiannis D, Vandenbroucke RE. Vandendriessche C, et al. Adv Drug Deliv Rev. 2022 Nov;190:114486. doi: 10.1016/j.addr.2022.114486. Epub 2022 Aug 8. Adv Drug Deliv Rev. 2022. PMID: 35952829 Free PMC article. Review.
Cited by
- New perspectives of the role of skeletal muscle derived extracellular vesicles in the pathogenesis of amyotrophic lateral sclerosis: the 'dying back' hypothesis.
Sbarigia C, Rome S, Dini L, Tacconi S. Sbarigia C, et al. J Extracell Biol. 2024 Nov 12;3(11):e70019. doi: 10.1002/jex2.70019. eCollection 2024 Nov. J Extracell Biol. 2024. PMID: 39534483 Free PMC article. Review. - Measurement of α-synuclein as protein cargo in plasma extracellular vesicles.
Gilboa T, Ter-Ovanesyan D, Wang SC, Whiteman S, Kannarkat GT, Church GM, Chen-Plotkin AS, Walt DR. Gilboa T, et al. Proc Natl Acad Sci U S A. 2024 Nov 5;121(45):e2408949121. doi: 10.1073/pnas.2408949121. Epub 2024 Oct 30. Proc Natl Acad Sci U S A. 2024. PMID: 39475636 - The plasma miRNAome in ADNI: Signatures to aid the detection of at-risk individuals.
Krüger DM, Pena-Centeno T, Liu S, Park T, Kaurani L, Pradhan R, Huang YN, Risacher SL, Burkhardt S, Schütz AL, Wan Y, Shaw LM, Brodsky AS, DeStefano AL, Lin H, Schroeder R, Krunic A, Hempel N, Sananbenesi F, Blusztajn JK, Saykin AJ, Delalle I, Nho K, Fischer A; Alzheimer's Disease Neuroimaging Initiative. Krüger DM, et al. Alzheimers Dement. 2024 Nov;20(11):7479-7494. doi: 10.1002/alz.14157. Epub 2024 Sep 18. Alzheimers Dement. 2024. PMID: 39291752 Free PMC article. - miR-92a-3p and miR-320a are Upregulated in Plasma Neuron-Derived Extracellular Vesicles of Patients with Frontotemporal Dementia.
Manzini V, Cappelletti P, Orefice NS, Brentari I, Rigby MJ, Lo Giudice M, Feligioni M, Rivabene R, Crestini A, Manfredi F, Talarico G, Bruno G, Corbo M, Puglielli L, Denti MA, Piscopo P. Manzini V, et al. Mol Neurobiol. 2024 Aug 14. doi: 10.1007/s12035-024-04386-z. Online ahead of print. Mol Neurobiol. 2024. PMID: 39138758 - Potential Early Effect Biomarkers for Ambient Air Pollution Related Mental Disorders.
Bai L, Wang K, Liu D, Wu S. Bai L, et al. Toxics. 2024 Jun 24;12(7):454. doi: 10.3390/toxics12070454. Toxics. 2024. PMID: 39058106 Free PMC article. Review.
References
- Arnold S. E., Hyman B. T., Flory J., Damasio A. R., Van Hoesen G. W. (1991). The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb. Cortex 1, 103–116. 10.1093/cercor/1.1.103 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases