The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice - PubMed (original) (raw)
The artificial sweetener acesulfame potassium affects the gut microbiome and body weight gain in CD-1 mice
Xiaoming Bian et al. PLoS One. 2017.
Abstract
Artificial sweeteners have been widely used in the modern diet, and their observed effects on human health have been inconsistent, with both beneficial and adverse outcomes reported. Obesity and type 2 diabetes have dramatically increased in the U.S. and other countries over the last two decades. Numerous studies have indicated an important role of the gut microbiome in body weight control and glucose metabolism and regulation. Interestingly, the artificial sweetener saccharin could alter gut microbiota and induce glucose intolerance, raising questions about the contribution of artificial sweeteners to the global epidemic of obesity and diabetes. Acesulfame-potassium (Ace-K), a FDA-approved artificial sweetener, is commonly used, but its toxicity data reported to date are considered inadequate. In particular, the functional impact of Ace-K on the gut microbiome is largely unknown. In this study, we explored the effects of Ace-K on the gut microbiome and the changes in fecal metabolic profiles using 16S rRNA sequencing and gas chromatography-mass spectrometry (GC-MS) metabolomics. We found that Ace-K consumption perturbed the gut microbiome of CD-1 mice after a 4-week treatment. The observed body weight gain, shifts in the gut bacterial community composition, enrichment of functional bacterial genes related to energy metabolism, and fecal metabolomic changes were highly gender-specific, with differential effects observed for males and females. In particular, ace-K increased body weight gain of male but not female mice. Collectively, our results may provide a novel understanding of the interaction between artificial sweeteners and the gut microbiome, as well as the potential role of this interaction in the development of obesity and the associated chronic inflammation.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
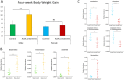
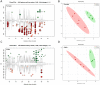
Fig 1. Effects of four weeks of Ace-K consumption on the body weight gain and gut microbiome composition of CD-1 mice.
(A) The body weight gain of Ace-K-treated male mice was significantly higher than that of the control male mice, while the body weight gain of female mice was not significantly different from that of the controls. (B) Ace-K consumption altered the composition of gut bacteria in female mice. The abundances of Lactobacillus, Clostridium, an unassigned Ruminococcaceae genus and an unassigned Oxalobacteraceae genus were significantly decreased, and the abundance of Mucispirillum was increased after Ace-K consumption. (C) Ace-K consumption altered the composition of gut bacteria in male mice. The abundances of Bacteroides, Anaerostipes and Sutterella were significantly increased after Ace-K consumption (*p<0.05, **p<0.01, ***p<0.001, N.S. p>0.05).
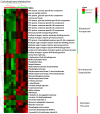
Fig 2. Functional gene enrichment analysis showing that functional genes related to carbohydrate metabolism were significantly decreased in Ace-K-treated female mice (p<0.05 for all genes listed here).
Fig 3. Functional gene enrichment analysis showing that functional genes related to carbohydrate metabolism were significantly increased in Ace-K-treated male mice (p<0.05).
Genes involved in carbohydrate transport (A), glycolysis and the TCA cycle (B), as well as carbohydrate degradation and fermentation (C), were consistently increased.
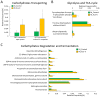
Fig 4. Multiple genes encoding pro-inflammatory mediators were significantly increased in male and female mice after Ace-K consumption (p<0.05).
Genes encoding LPS metabolism proteins (A) and flagella components (B) were increased in Ace-K-treated female mice. Genes encoding LPS metabolism proteins (C) and thiol-activated cytolysin (D) were increased in Ace-K-treated male mice.
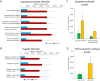
Fig 5. Ace-K consumption changed the fecal metabolome of female (A, B) and male (C, D) mice, as illustrated by the cloud and PLS-DA plots.
Fig 6. Ace-K consumption significantly altered key fecal metabolites in female (A) and male (B) mice (*p<0.05, **p<0.01).
Similar articles
- Effects of the Artificial Sweetener Neotame on the Gut Microbiome and Fecal Metabolites in Mice.
Chi L, Bian X, Gao B, Tu P, Lai Y, Ru H, Lu K. Chi L, et al. Molecules. 2018 Feb 9;23(2):367. doi: 10.3390/molecules23020367. Molecules. 2018. PMID: 29425148 Free PMC article. - Investigating the gut microbiome and metabolome following treatment with artificial sweeteners acesulfame potassium and saccharin in young adult Wistar rats.
Murali A, Giri V, Cameron HJ, Sperber S, Zickgraf FM, Haake V, Driemert P, Walk T, Kamp H, Rietjens IM, van Ravenzwaay B. Murali A, et al. Food Chem Toxicol. 2022 Jul;165:113123. doi: 10.1016/j.fct.2022.113123. Epub 2022 May 16. Food Chem Toxicol. 2022. PMID: 35588986 - The Association Between Artificial Sweeteners and Obesity.
Pearlman M, Obert J, Casey L. Pearlman M, et al. Curr Gastroenterol Rep. 2017 Nov 21;19(12):64. doi: 10.1007/s11894-017-0602-9. Curr Gastroenterol Rep. 2017. PMID: 29159583 Review. - High-intensity sweetener consumption and gut microbiome content and predicted gene function in a cross-sectional study of adults in the United States.
Frankenfeld CL, Sikaroodi M, Lamb E, Shoemaker S, Gillevet PM. Frankenfeld CL, et al. Ann Epidemiol. 2015 Oct;25(10):736-42.e4. doi: 10.1016/j.annepidem.2015.06.083. Epub 2015 Jul 17. Ann Epidemiol. 2015. PMID: 26272781 - Non-caloric artificial sweeteners and the microbiome: findings and challenges.
Suez J, Korem T, Zilberman-Schapira G, Segal E, Elinav E. Suez J, et al. Gut Microbes. 2015;6(2):149-55. doi: 10.1080/19490976.2015.1017700. Epub 2015 Apr 1. Gut Microbes. 2015. PMID: 25831243 Free PMC article. Review.
Cited by
- Non-nutritive sweeteners and their impacts on the gut microbiome and host physiology.
Richardson IL, Frese SA. Richardson IL, et al. Front Nutr. 2022 Aug 25;9:988144. doi: 10.3389/fnut.2022.988144. eCollection 2022. Front Nutr. 2022. PMID: 36091255 Free PMC article. Review. - Ultra-processed foods and food additives in gut health and disease.
Whelan K, Bancil AS, Lindsay JO, Chassaing B. Whelan K, et al. Nat Rev Gastroenterol Hepatol. 2024 Jun;21(6):406-427. doi: 10.1038/s41575-024-00893-5. Epub 2024 Feb 22. Nat Rev Gastroenterol Hepatol. 2024. PMID: 38388570 Review. - The Effects of Artificial Sweeteners on Intestinal Nutrient-Sensing Receptors: Dr. Jekyll or Mr. Hyde?
Posta E, Fekete I, Gyarmati E, Stündl L, Zold E, Barta Z. Posta E, et al. Life (Basel). 2023 Dec 20;14(1):10. doi: 10.3390/life14010010. Life (Basel). 2023. PMID: 38276259 Free PMC article. Review. - Consumption of Non-Nutritive Sweetener, Acesulfame Potassium Exacerbates Atherosclerosis through Dysregulation of Lipid Metabolism in ApoE-/- Mice.
Lin CH, Li HY, Wang SH, Chen YH, Chen YC, Wu HT. Lin CH, et al. Nutrients. 2021 Nov 9;13(11):3984. doi: 10.3390/nu13113984. Nutrients. 2021. PMID: 34836239 Free PMC article. - Artificial sweetener is a growing threat for metabolic syndrome: why is extra attention required?
Sabarathinam S, Dhanasekaran D, Ganamurali N. Sabarathinam S, et al. Future Sci OA. 2023 Jun 23;9(8):FSO880. doi: 10.2144/fsoa-2023-0092. eCollection 2023 Sep. Future Sci OA. 2023. PMID: 37621846 Free PMC article. No abstract available.
References
- Gardner C, Wylie-Rosett J, Gidding SS, Steffen LM, Johnson RK, Reader D, et al. Nonnutritive sweeteners: current use and health perspectives a scientific statement from the American heart association and the American diabetes association. Diabetes Care. 2012;35(8):1798–808. 10.2337/dc12-9002 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous