The Therapeutic Benefit of Bacterial Membrane Vesicles - PubMed (original) (raw)
Review
The Therapeutic Benefit of Bacterial Membrane Vesicles
Natalie J Bitto et al. Int J Mol Sci. 2017.
Abstract
The therapeutic potential of extracellular vesicles from eukaryotes has gained strong interest in recent years. However, research into the therapeutic application of their bacterial counterparts, known as bacterial membrane vesicles, is only just beginning to be appreciated. Membrane vesicles (MVs) from both Gram-positive and Gram-negative bacteria offer significant advantages in therapeutic development, including large-scale, cost effective production and ease of molecular manipulation to display foreign antigens. The nanoparticle size of MVs enables their dissemination through numerous tissue types, and their natural immunogenicity and self-adjuvanting capability can be harnessed to induce both cell-mediated and humoral immunity in vaccine design. Moreover, the ability to target MVs to specific tissues through the display of surface receptors raises their potential use as targeted MV-based anti-cancer therapy. This review discusses recent advances in MV research with particular emphasis on exciting new possibilities for the application of MVs in therapeutic design.
Keywords: bacterial membrane vesicles; cancer therapy; recombinant bacterial membrane vesicles; vaccine design.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
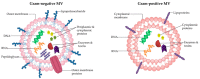
Figure 1
Architecture and composition of Gram-negative and Gram-positive MVs. While MVs are heterogeneous in size and contents, typically Gram-negative MVs are encased in the outer membrane which is embedded with LPS and outer-membrane proteins, and carry periplasmic contents including peptidoglycan, enzymes and toxins, as well as cytoplasmic proteins and nucleic acids. Gram-positive MVs are comprised of the cytoplasmic membrane and have been reported to contain lipoprotein, cytoplasmic proteins, enzymes, toxins and nucleic acids.
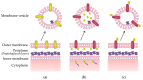
Figure 2
Methods of packaging recombinant proteins into MVs. (a) Overexpression of a recombinant membrane protein (yellow) may lead to enrichment on the MV surface; (b) A periplasmic signal sequence (blue) can be used to direct the protein of interest (yellow) into the periplasm, whereby the protein of interest may become encapsulated into budding MVs; (c) Fusion of a protein of interest (yellow) with a membrane protein can lead to targeted display on the surface of MVs.
Similar articles
- Bacterial membrane vesicles combined with nanoparticles for bacterial vaccines and cancer immunotherapy.
Xu W, Maruyama S, Sato A, Niidome T. Xu W, et al. Colloids Surf B Biointerfaces. 2024 Nov;243:114125. doi: 10.1016/j.colsurfb.2024.114125. Epub 2024 Jul 25. Colloids Surf B Biointerfaces. 2024. PMID: 39079185 Review. - Fight bacteria with bacteria: Bacterial membrane vesicles as vaccines and delivery nanocarriers against bacterial infections.
Gan Y, Li C, Peng X, Wu S, Li Y, Tan JPK, Yang YY, Yuan P, Ding X. Gan Y, et al. Nanomedicine. 2021 Jul;35:102398. doi: 10.1016/j.nano.2021.102398. Epub 2021 Apr 24. Nanomedicine. 2021. PMID: 33901646 Review. - Bacterial Membrane Vesicles in Pneumonia: From Mediators of Virulence to Innovative Vaccine Candidates.
Behrens F, Funk-Hilsdorf TC, Kuebler WM, Simmons S. Behrens F, et al. Int J Mol Sci. 2021 Apr 8;22(8):3858. doi: 10.3390/ijms22083858. Int J Mol Sci. 2021. PMID: 33917862 Free PMC article. Review. - Bacterial membrane vesicles as promising vaccine candidates.
Jiang L, Schinkel M, van Essen M, Schiffelers RM. Jiang L, et al. Eur J Pharm Biopharm. 2019 Dec;145:1-6. doi: 10.1016/j.ejpb.2019.09.021. Epub 2019 Sep 24. Eur J Pharm Biopharm. 2019. PMID: 31560955 Review. - Immunomodulatory roles and novel applications of bacterial membrane vesicles.
Gilmore WJ, Johnston EL, Zavan L, Bitto NJ, Kaparakis-Liaskos M. Gilmore WJ, et al. Mol Immunol. 2021 Jun;134:72-85. doi: 10.1016/j.molimm.2021.02.027. Epub 2021 Mar 13. Mol Immunol. 2021. PMID: 33725501 Review.
Cited by
- Dual roles of the conditional extracellular vesicles derived from Pseudomonas aeruginosa biofilms: Promoting and inhibiting bacterial biofilm growth.
Saad MG, Beyenal H, Dong WJ. Saad MG, et al. Biofilm. 2024 Feb 6;7:100183. doi: 10.1016/j.bioflm.2024.100183. eCollection 2024 Jun. Biofilm. 2024. PMID: 38380422 Free PMC article. - Bacterial extracellular vesicle applications in cancer immunotherapy.
Suri K, D'Souza A, Huang D, Bhavsar A, Amiji M. Suri K, et al. Bioact Mater. 2022 Oct 31;22:551-566. doi: 10.1016/j.bioactmat.2022.10.024. eCollection 2023 Apr. Bioact Mater. 2022. PMID: 36382022 Free PMC article. - _Helicobacter pylori_-Derived Outer Membrane Vesicles (OMVs): Role in Bacterial Pathogenesis?
Jarzab M, Posselt G, Meisner-Kober N, Wessler S. Jarzab M, et al. Microorganisms. 2020 Aug 31;8(9):1328. doi: 10.3390/microorganisms8091328. Microorganisms. 2020. PMID: 32878302 Free PMC article. Review. - Evolution of Vaccines Formulation to Tackle the Challenge of Anti-Microbial Resistant Pathogens.
Tognetti F, Biagini M, Denis M, Berti F, Maione D, Stranges D. Tognetti F, et al. Int J Mol Sci. 2023 Jul 27;24(15):12054. doi: 10.3390/ijms241512054. Int J Mol Sci. 2023. PMID: 37569427 Free PMC article. Review. - Combining Protein Ligation Systems to Expand the Functionality of Semi-Synthetic Outer Membrane Vesicle Nanoparticles.
van den Berg van Saparoea HB, Houben D, Kuijl C, Luirink J, Jong WSP. van den Berg van Saparoea HB, et al. Front Microbiol. 2020 May 12;11:890. doi: 10.3389/fmicb.2020.00890. eCollection 2020. Front Microbiol. 2020. PMID: 32477305 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources