Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans - PubMed (original) (raw)
Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis following Resistance Exercise in Humans
Sarah R Jackman et al. Front Physiol. 2017.
Abstract
The ingestion of intact protein or essential amino acids (EAA) stimulates mechanistic target of rapamycin complex-1 (mTORC1) signaling and muscle protein synthesis (MPS) following resistance exercise. The purpose of this study was to investigate the response of myofibrillar-MPS to ingestion of branched-chain amino acids (BCAAs) only (i.e., without concurrent ingestion of other EAA, intact protein, or other macronutrients) following resistance exercise in humans. Ten young (20.1 ± 1.3 years), resistance-trained men completed two trials, ingesting either 5.6 g BCAA or a placebo (PLA) drink immediately after resistance exercise. Myofibrillar-MPS was measured during exercise recovery with a primed, constant infusion of L-[ring13C6] phenylalanine and collection of muscle biopsies pre and 4 h-post drink ingestion. Blood samples were collected at time-points before and after drink ingestion. Western blotting was used to measure the phosphorylation status of mTORC1 signaling proteins in biopsies collected pre, 1-, and 4 h-post drink. The percentage increase from baseline in plasma leucine (300 ± 96%), isoleucine (300 ± 88%), and valine (144 ± 59%) concentrations peaked 0.5 h-post drink in BCAA. A greater phosphorylation status of S6K1Thr389 (P = 0.017) and PRAS40 (P = 0.037) was observed in BCAA than PLA at 1 h-post drink ingestion. Myofibrillar-MPS was 22% higher (P = 0.012) in BCAA (0.110 ± 0.009%/h) than PLA (0.090 ± 0.006%/h). Phenylalanine Ra was ~6% lower in BCAA (18.00 ± 4.31 μmol·kgBM-1) than PLA (21.75 ± 4.89 μmol·kgBM-1; P = 0.028) after drink ingestion. We conclude that ingesting BCAAs alone increases the post-exercise stimulation of myofibrillar-MPS and phosphorylation status mTORC1 signaling.
Keywords: amino acid ingestion; fractional synthesis rate; intracellular signaling proteins; leucine; muscle anabolism.
Figures
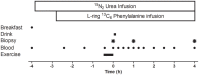
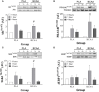
Figure 1
Schematic diagram of the infusion protocol. A baseline blood sample was collected before participants consumed an energy-rich, high-protein breakfast. A bout of unilateral leg-resistance exercise was performed 3 h after breakfast. Muscle biopsies (vastus lateralis) were collected from the exercised leg immediately prior (0 h), 1-, or 4 h-post drink ingestion. Drink ingestion was either a branched-chain amino acid containing beverage (BCAA) or placebo (PLA). Multiple blood samples were collected throughout the protocol. Ex, exercise.
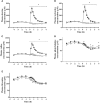
Figure 2
Plasma concentrations of (A) leucine, (B) isoleucine, (C) valine, (D) phenylalanine, and (E) threonine pre and post ingestion of either a branched-chain amino acid containing drink (BCAA, closed circles) or placebo drink (PLA, open circles) following resistance exercise. Data are displayed as means ± SE. *Significant difference between trials (P < 0.05).
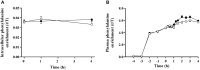
Figure 3
Muscle intracellular (A) and plasma (B) 13C6 phenylalanine enrichments pre and post ingestion of either a branched-chain amino acid containing drink (BCAA, black circles) or placebo drink (PLA, white circles) following resistance exercise. Data are displayed as means ± SE.
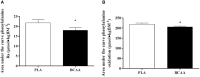
Figure 4
Phenylalanine kinetics, expressed as, total area under the curve for phenylalanine rate of appearance (A) and total area under the curve for phenylalanine oxidation (B) following the post-exercise ingestion of a branched-chain amino acid (BCAA, black bars) or placebo drink (PLA, white bars). Data are displayed as means ± SE. *Significant difference compared with PLA (P < 0.05).
Figure 5
Phosphorylation status of, AKTSer473 (A), PRAS40Thr246 (B), S6K1Thr389 (C), and 4EBP1Thr37/45 (D) immediately pre (0 h), 1-, and 4 h-post ingestion of either a branched-chain amino acid containing drink (BCAA, black bars) or placebo drink (PLA, white bars) following resistance exercise. Data are displayed as means ± SE. #Significant difference from 0 h in respective trials (P < 0.05).
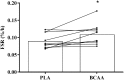
Figure 6
Muscle myofibrillar fractional synthesis rate following the post-exercise ingestion of a branched-chain amino acid containing drink (BCAA) or placebo drink (PLA). Data are displayed as means (bars) and individual responses (dots and lines). *Significant difference compared with PLA (P < 0.05).
Similar articles
- Co-Ingestion of Branched-Chain Amino Acids and Carbohydrate Stimulates Myofibrillar Protein Synthesis Following Resistance Exercise in Trained Young Men.
Jackman SR, Wallis GA, Yu J, Philp A, Baar K, Tipton KD, Witard OC. Jackman SR, et al. Int J Sport Nutr Exerc Metab. 2023 May 24;33(4):189-197. doi: 10.1123/ijsnem.2023-0015. Print 2023 Jul 1. Int J Sport Nutr Exerc Metab. 2023. PMID: 37225168 - Branched-chain amino acid and branched-chain ketoacid ingestion increases muscle protein synthesis rates in vivo in older adults: a double-blind, randomized trial.
Fuchs CJ, Hermans WJH, Holwerda AM, Smeets JSJ, Senden JM, van Kranenburg J, Gijsen AP, Wodzig WKHW, Schierbeek H, Verdijk LB, van Loon LJC. Fuchs CJ, et al. Am J Clin Nutr. 2019 Oct 1;110(4):862-872. doi: 10.1093/ajcn/nqz120. Am J Clin Nutr. 2019. PMID: 31250889 Free PMC article. Clinical Trial. - Activation of mTORC1 by leucine is potentiated by branched-chain amino acids and even more so by essential amino acids following resistance exercise.
Moberg M, Apró W, Ekblom B, van Hall G, Holmberg HC, Blomstrand E. Moberg M, et al. Am J Physiol Cell Physiol. 2016 Jun 1;310(11):C874-84. doi: 10.1152/ajpcell.00374.2015. Epub 2016 Apr 6. Am J Physiol Cell Physiol. 2016. PMID: 27053525 Clinical Trial. - The effects of branched-chain amino acids on muscle protein synthesis, muscle protein breakdown and associated molecular signalling responses in humans: an update.
Kaspy MS, Hannaian SJ, Bell ZW, Churchward-Venne TA. Kaspy MS, et al. Nutr Res Rev. 2024 Dec;37(2):273-286. doi: 10.1017/S0954422423000197. Epub 2023 Sep 8. Nutr Res Rev. 2024. PMID: 37681443 Review. - International society of sports nutrition position stand: nutrient timing.
Kerksick CM, Arent S, Schoenfeld BJ, Stout JR, Campbell B, Wilborn CD, Taylor L, Kalman D, Smith-Ryan AE, Kreider RB, Willoughby D, Arciero PJ, VanDusseldorp TA, Ormsbee MJ, Wildman R, Greenwood M, Ziegenfuss TN, Aragon AA, Antonio J. Kerksick CM, et al. J Int Soc Sports Nutr. 2017 Aug 29;14:33. doi: 10.1186/s12970-017-0189-4. eCollection 2017. J Int Soc Sports Nutr. 2017. PMID: 28919842 Free PMC article. Review.
Cited by
- Branched-chain amino acids: physico-chemical properties, industrial synthesis and role in signaling, metabolism and energy production.
Reifenberg P, Zimmer A. Reifenberg P, et al. Amino Acids. 2024 Aug 28;56(1):51. doi: 10.1007/s00726-024-03417-2. Amino Acids. 2024. PMID: 39198298 Free PMC article. Review. - The Combination of Lactoferrin and Creatine Ameliorates Muscle Decay in a Sarcopenia Murine Model.
Wu W, Guo X, Qu T, Huang Y, Tao J, He J, Wang X, Luo J, An P, Zhu Y, Sun Y, Luo Y. Wu W, et al. Nutrients. 2024 Jun 19;16(12):1958. doi: 10.3390/nu16121958. Nutrients. 2024. PMID: 38931310 Free PMC article. - Influence of Amino Acids and Exercise on Muscle Protein Turnover, Particularly in Cancer Cachexia.
Pradhan R, Dieterich W, Natarajan A, Schwappacher R, Reljic D, Herrmann HJ, Neurath MF, Zopf Y. Pradhan R, et al. Cancers (Basel). 2024 May 18;16(10):1921. doi: 10.3390/cancers16101921. Cancers (Basel). 2024. PMID: 38791998 Free PMC article. Review. - Reconsidering the pre-eminence of dietary leucine and plasma leucinemia for predicting the stimulation of postprandial muscle protein synthesis rates.
Monteyne AJ, West S, Stephens FB, Wall BT. Monteyne AJ, et al. Am J Clin Nutr. 2024 Jul;120(1):7-16. doi: 10.1016/j.ajcnut.2024.04.032. Epub 2024 May 3. Am J Clin Nutr. 2024. PMID: 38705358 Free PMC article. - Chilean Market Protein Shakes Composition.
Jorquera C, Droppelmann G, Pridal P, Faúndez J, Feijoo F. Jorquera C, et al. Nutrients. 2024 Apr 11;16(8):1129. doi: 10.3390/nu16081129. Nutrients. 2024. PMID: 38674821 Free PMC article.
References
- Anthony J. C., Anthony T. G., Kimball S. R., Vary T. C., Jefferson L. S. (2000). Orally administered leucine stimulates protein synthesis in skeletal muscle of postabsorptive rats in association with increased eIF4F formation. J. Nutr. 130, 139–145. - PubMed
- Anthony J. C., Anthony T. G., Layman D. K. (1999). Leucine supplementation enhances skeletal muscle recovery in rats following exercise. J. Nutr. 129, 1102–1106. - PubMed
- Atherton P. J., Etheridge T., Watt P. W., Wilkinson D., Selby A., Rankin D., et al. . (2010a). Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am. J. Clin. Nutr. 92, 1080–1088. 10.3945/ajcn.2010.29819 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous