The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro - PubMed (original) (raw)
The alkaloids of Banisteriopsis caapi, the plant source of the Amazonian hallucinogen Ayahuasca, stimulate adult neurogenesis in vitro
Jose A Morales-García et al. Sci Rep. 2017.
Abstract
Banisteriopsis caapi is the basic ingredient of ayahuasca, a psychotropic plant tea used in the Amazon for ritual and medicinal purposes, and by interested individuals worldwide. Animal studies and recent clinical research suggests that B. caapi preparations show antidepressant activity, a therapeutic effect that has been linked to hippocampal neurogenesis. Here we report that harmine, tetrahydroharmine and harmaline, the three main alkaloids present in B. caapi, and the harmine metabolite harmol, stimulate adult neurogenesis in vitro. In neurospheres prepared from progenitor cells obtained from the subventricular and the subgranular zones of adult mice brains, all compounds stimulated neural stem cell proliferation, migration, and differentiation into adult neurons. These findings suggest that modulation of brain plasticity could be a major contribution to the antidepressant effects of ayahuasca. They also expand the potential application of B. caapi alkaloids to other brain disorders that may benefit from stimulation of endogenous neural precursor niches.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
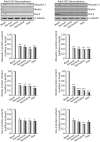
Effects of ayahuasca β-carboline alkaloids on stemness of cultured adult neurospheres. Representative Western blots and bar graphs showing expression levels of the precursor cell markers musashi-1, nestin and SOX-2 after treatment with each of the four alkaloids tested (1 µM). Values in bar graphs indicate mean ± SD of the quantification of at least three independent experiments corresponding to four different cellular pools. The left side of the image shows results for the subventricular zone (SVZ) of the brain. The right side of the image shows results for the subgranular zone of the hippocampus (SGZ). *p ≤ 0.05; **P ≤ 0.01; ***p ≤ 0.001 indicate significant results in the post-hoc pair-wise comparisons (Bonferroni) versus non-treated (basal) cultures.
Figure 2
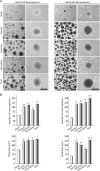
Effects of ayahuasca β-carboline alkaloids on adult neurosphere formation. (a) Representative phase-contrast micrographs showing the number and size of neurospheres after 7 days in culture in the presence of each of the four alkaloids tested (1 µM). The number and diameter of at least 50 neurospheres was determined in control and treated cultures. Scale bar = 100 μm. (b) Bar graphs showing results as mean values ± SD of the quantification of at least three independent experiments corresponding to four different cellular pools. The left side of the image shows results for the subventricular zone (SVZ) of the brain. The right side of the image shows results for he subgranular zone of the hippocampus (SGZ). *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 indicate significant results in the post-hoc pair-wise comparisons (Bonferroni) versus non-treated (basal) cultures.
Figure 3
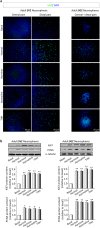
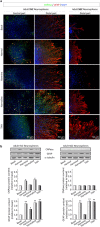
Effects of ayahuasca β-carboline alkaloids on adult neural stem cells proliferation. (a) Representative immunofluorescence images showing the expression of the cellular marker for proliferation ki67 (green) in neurospheres derived from the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone (SGZ) of the hippocampus. SVZ-derived neurospheres are shown in two panels showing the central part of the sphere (left) and the distal migration site (right). Single images from SGZ-derived neurospheres show the whole neurosphere, including the central and distal areas. DAPI was used for nuclear staining. Scale bar = 50 μm. (b) Representative Western blots of ki67 and the proliferating cell nuclear antigen (PCNA) levels in neurospheres treated for 7 days with each of the four alkaloids tested (1 µM). Bar graphs show the results of the quantification analyses. Each bar indicates relative protein levels expressed as mean ± SD of the quantification of at least three independent experiments corresponding to four different cellular pools. The left side of the image shows results for the subventricular zone (SVZ) of the brain. The right side of the image shows results for he subgranular zone of the hippocampus (SGZ). *p ≤ 0.05; **p ≤ 0.01 indicate significant results in the post-hoc pair-wise comparisons (Bonferroni) versus non-treated (basal) cultures.
Figure 4
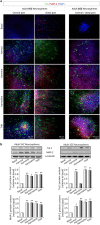
Ayahuasca β-carboline alkaloids promote stem cell differentiation towards a neuronal phenotype. After 7 days on culture in the presence of harmol, harmine, harmaline and tetrahydroharmine (THH), free floating neurospheres derived from the adult subgranular (SGZ) and subventricular (SVZ) zone were adhered on coated coverslips and allowed to differentiate for 3 days in the presence of alkaloids at 1 µM. (a) Representative immunofluorescence images showing the expression of the neuronal markers β-III-Tubulin (TuJ-1 clone, green) and MAP-2 (red) in neurospheres. DAPI was used for nuclear staining. SVZ-derived neurospheres are shown in two panels showing the central part of the sphere (left) and the distal migration site (right). Single images from SGZ-derived neurospheres show the whole neurosphere, including the central and distal areas. Scale bar = 50 μm. (b) Representative Western blots of β-tubulin and MAP-2. Quantification analyses are also shown. Results are the mean ± SD of the quantification of at least three independent experiments corresponding to four different cellular pools. The left side of the image shows results for the subventricular zone (SVZ) of the brain. The right side of the image shows results for he subgranular zone of the hippocampus (SGZ). *p ≤ 0.05; **p ≤ 0.01 indicate significant results in the post-hoc pair-wise comparisons (Bonferroni) versus non-treated (basal) cultures.
Figure 5
Ayahuasca β-carboline alkaloids promote astrogliogenesis. Neurospheres derived from the adult subgranular (SGZ) and subventricular (SVZ) zone were cultured in the presence of harmol, harmine, harmaline and tetrahydroharmine (THH). After 7 days neurospheres were adhered on coated coverslips and allowed to differentiate for 3 days in the presence of alkaloids at 1 µM. (a) Neurosphere immunofluorescence images showing in green the expression of CNPase (oligodendrocyte marker) and glial fibrillary acidic protein (GFAP, red) that stains astrocytes. SVZ-derived neurospheres are shown in two panels showing the central part of the neurosphere (left) and the distal migration site (right). Single images from SGZ-derived neurospheres show the distal part of the neurosphere. DAPI was used for nuclear staining. Scale bar = 50 μm. (b) Representative Western blots of CNPase and GFAP. Quantification analyses are also shown. Results are the mean ± SD of the quantification of at least three independent experiments corresponding to four different cellular pools. The left side of the image shows results for the subventricular zone (SVZ) of the brain. The right side of the image shows results for he subgranular zone of the hippocampus (SGZ). *p ≤ 0.05; **p ≤ 0.01 indicate significant results in the post-hoc pair-wise comparisons (Bonferroni) versus non-treated (basal) cultures.
Similar articles
- Composition, standardization and chemical profiling of Banisteriopsis caapi, a plant for the treatment of neurodegenerative disorders relevant to Parkinson's disease.
Wang YH, Samoylenko V, Tekwani BL, Khan IA, Miller LS, Chaurasiya ND, Rahman MM, Tripathi LM, Khan SI, Joshi VC, Wigger FT, Muhammad I. Wang YH, et al. J Ethnopharmacol. 2010 Apr 21;128(3):662-71. doi: 10.1016/j.jep.2010.02.013. Epub 2010 Feb 26. J Ethnopharmacol. 2010. PMID: 20219660 Free PMC article. - Mutagenicity of Ayahuasca and Their Constituents to the Salmonella/Microsome Assay.
Kummrow F, Maselli BS, Lanaro R, Costa JL, Umbuzeiro GA, Linardi A. Kummrow F, et al. Environ Mol Mutagen. 2019 Apr;60(3):269-276. doi: 10.1002/em.22263. Epub 2018 Nov 29. Environ Mol Mutagen. 2019. PMID: 30488498 - Metabolism and disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca.
Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Riba J, et al. Drug Test Anal. 2012 Jul-Aug;4(7-8):610-6. doi: 10.1002/dta.1344. Epub 2012 Apr 19. Drug Test Anal. 2012. PMID: 22514127 - Effects of the Natural β-Carboline Alkaloid Harmine, a Main Constituent of Ayahuasca, in Memory and in the Hippocampus: A Systematic Literature Review of Preclinical Studies.
Dos Santos RG, Hallak JE. Dos Santos RG, et al. J Psychoactive Drugs. 2017 Jan-Mar;49(1):1-10. doi: 10.1080/02791072.2016.1260189. Epub 2016 Dec 5. J Psychoactive Drugs. 2017. PMID: 27918874 Review. - Toxicokinetics and Toxicodynamics of Ayahuasca Alkaloids N,_N_-Dimethyltryptamine (DMT), Harmine, Harmaline and Tetrahydroharmine: Clinical and Forensic Impact.
Brito-da-Costa AM, Dias-da-Silva D, Gomes NGM, Dinis-Oliveira RJ, Madureira-Carvalho Á. Brito-da-Costa AM, et al. Pharmaceuticals (Basel). 2020 Oct 23;13(11):334. doi: 10.3390/ph13110334. Pharmaceuticals (Basel). 2020. PMID: 33114119 Free PMC article. Review.
Cited by
- A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus.
Lima da Cruz RV, Moulin TC, Petiz LL, Leão RN. Lima da Cruz RV, et al. Front Mol Neurosci. 2018 Sep 4;11:312. doi: 10.3389/fnmol.2018.00312. eCollection 2018. Front Mol Neurosci. 2018. PMID: 30233313 Free PMC article. - Corrigendum: A Single Dose of 5-MeO-DMT Stimulates Cell Proliferation, Neuronal Survivability, Morphological and Functional Changes in Adult Mice Ventral Dentate Gyrus.
Lima da Cruz RV, Moulin TC, Petiz LL, Leão RN. Lima da Cruz RV, et al. Front Mol Neurosci. 2019 Apr 4;12:79. doi: 10.3389/fnmol.2019.00079. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31019450 Free PMC article. - The effects of ketamine and classic hallucinogens on neurotrophic and inflammatory markers in unipolar treatment-resistant depression: a systematic review of clinical trials.
Rossi GN, Hallak JEC, Baker G, Dursun SM, Dos Santos RG. Rossi GN, et al. Eur Arch Psychiatry Clin Neurosci. 2023 Feb;273(1):129-155. doi: 10.1007/s00406-022-01460-2. Epub 2022 Jul 13. Eur Arch Psychiatry Clin Neurosci. 2023. PMID: 35829812 Review. - Monoamine Oxidase Inhibition by Plant-Derived β-Carbolines; Implications for the Psychopharmacology of Tobacco and Ayahuasca.
Berlowitz I, Egger K, Cumming P. Berlowitz I, et al. Front Pharmacol. 2022 May 2;13:886408. doi: 10.3389/fphar.2022.886408. eCollection 2022. Front Pharmacol. 2022. PMID: 35600851 Free PMC article. Review. - Parkinson's Disease Master Regulators on Substantia Nigra and Frontal Cortex and Their Use for Drug Repositioning.
Vargas DM, De Bastiani MA, Parsons RB, Klamt F. Vargas DM, et al. Mol Neurobiol. 2021 Apr;58(4):1517-1534. doi: 10.1007/s12035-020-02203-x. Epub 2020 Nov 19. Mol Neurobiol. 2021. PMID: 33211252
References
- Schultes, R. E. The botany and chemistry of hallucinogens. (Thomas, 1980).
- McKenna, D. & Riba, J. New World Tryptamine Hallucinogens and the Neuroscience of Ayahuasca. Curr. Top. Behav. Neurosci. doi:10.1007/7854_2016_472 (2017). - PubMed
- Riba, J. Human Pharmacology of Ayahuasca. (Autonomous University of Barcelona, 2003). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources