Poly(Dopamine)-Assisted Immobilization of Xu Duan on 3D Printed Poly(Lactic Acid) Scaffolds to Up-Regulate Osteogenic and Angiogenic Markers of Bone Marrow Stem Cells - PubMed (original) (raw)
Poly(Dopamine)-Assisted Immobilization of Xu Duan on 3D Printed Poly(Lactic Acid) Scaffolds to Up-Regulate Osteogenic and Angiogenic Markers of Bone Marrow Stem Cells
Chia-Hung Yeh et al. Materials (Basel). 2015.
Abstract
Three-dimensional printing is a versatile technique to generate large quantities of a wide variety of shapes and sizes of polymer. The aim of this study is to develop functionalized 3D printed poly(lactic acid) (PLA) scaffolds and use a mussel-inspired surface coating and Xu Duan (XD) immobilization to regulate cell adhesion, proliferation and differentiation of human bone-marrow mesenchymal stem cells (hBMSCs). We prepared PLA scaffolds and coated with polydopamine (PDA). The chemical composition and surface properties of PLA/PDA/XD were characterized by XPS. PLA/PDA/XD controlled hBMSCs' responses in several ways. Firstly, adhesion and proliferation of hBMSCs cultured on PLA/PDA/XD were significantly enhanced relative to those on PLA. In addition, the focal adhesion kinase (FAK) expression of cells was increased and promoted cell attachment depended on the XD content. In osteogenesis assay, the osteogenesis markers of hBMSCs cultured on PLA/PDA/XD were significantly higher than seen in those cultured on a pure PLA/PDA scaffolds. Moreover, hBMSCs cultured on PLA/PDA/XD showed up-regulation of the ang-1 and vWF proteins associated with angiogenic differentiation. Our results demonstrate that the bio-inspired coating synthetic PLA polymer can be used as a simple technique to render the surfaces of synthetic scaffolds active, thus enabling them to direct the specific responses of hBMSCs.
Keywords: 3D printed-scaffold; angiogenic; dopamine; osteogenic; poly (lactic acid); tissue engineering.
Conflict of interest statement
The authors declare no conflict of interest. No benefit of any kind has been or will be received either directly or indirectly by the authors.
Figures
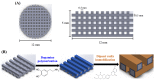
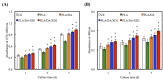
Figure 1
The (A) top view and side view of 3D printed poly(lactic acid) (PLA) scaffold; (B) Schematic illustration of dopamine-assisted immobilization of XD on surfaces.
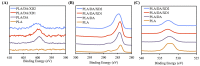
Figure 2
XPS (A) N1s; (B) C1s; and (C) O1s high-resolution spectra obtained on PLA scaffolds after coating with dopamine.
Figure 3
SEM images of PLA scaffold coated with DA and XD.
Figure 4
Release profile of XD from PLA scaffolds in DMEM for (A) short and (B) long times. The values shown are means ± standard errors for all the assays.
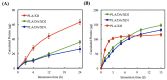
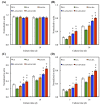
Figure 5
The adhesion of hBMSCs cultured with various specimens for different time points. The values shown are means ± standard errors for all the assays. “#” indicates a significant difference (p < 0.05) compared to PLA; “*” indicates a significant difference (p < 0.05) compared to PLA/DA.
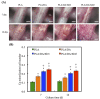
Figure 6
The morphology of hBMSCs adhered on PLA/PDA/XD scaffolds for 3 and 24 h.
Figure 7
The pFAK expression of hBMSCs cultured on various specimens for 3 h. The values shown are means ± standard errors for all the assays. “#” indicates a significant difference (p < 0.05) compared to PLA; “*” indicates a significant difference (p < 0.05) compared to PLA/DA.
Figure 8
(A) PrestoBlue® assay and (B) LDH assay of hBMSCs cultured on various specimens for different time points. The values shown are means ± standard errors for all the assays. “#” indicates a significant difference (p < 0.05) compared to PLA; “*” indicates a significant difference (p < 0.05) compared to PLA/DA.
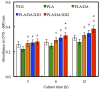
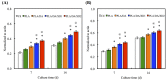
Figure 9
(A) Col; (B) ALP; (C) BSP and (D) OC gene expression in the hBMSCs were cultured on the various specimens for 7 and 14 days. The values shown are means ± standard errors for all the assays. “@” indicates a significant difference (p < 0.05) compared to PLA; “*” indicates a significant difference (p < 0.05) compared to PLA/DA.
Figure 10
(A) Alizarin Red S staining and (B) quantification of calcium mineral deposits of hBMSCs cultured on various scaffolds for 7 and 14 days. The values shown are means ± standard errors for all the assays. “*” indicates a significant difference (p < 0.05) compared to PLA; “@” indicates a significant difference (p < 0.05) compared to PLA/DA.
Figure 11
The protein expression of (A) Ang-1 and (B) vWF of hBMSCs cultured on scaffolds for different days. The values shown are means ± standard errors for all the assays. “*” indicates a significant difference (p < 0.05) compared to PLA; “@” indicates a significant difference (p < 0.05) compared to PLA/DA.
Similar articles
- Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering.
Kao CT, Lin CC, Chen YW, Yeh CH, Fang HY, Shie MY. Kao CT, et al. Mater Sci Eng C Mater Biol Appl. 2015 Nov 1;56:165-73. doi: 10.1016/j.msec.2015.06.028. Epub 2015 Jun 17. Mater Sci Eng C Mater Biol Appl. 2015. PMID: 26249577 - Osteogenesis of human adipose-derived stem cells on poly(dopamine)-coated electrospun poly(lactic acid) fiber mats.
Lin CC, Fu SJ. Lin CC, et al. Mater Sci Eng C Mater Biol Appl. 2016 Jan 1;58:254-63. doi: 10.1016/j.msec.2015.08.009. Epub 2015 Aug 11. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26478309 - Development of mussel-inspired 3D-printed poly (lactic acid) scaffold grafted with bone morphogenetic protein-2 for stimulating osteogenesis.
Cheng CH, Chen YW, Kai-Xing Lee A, Yao CH, Shie MY. Cheng CH, et al. J Mater Sci Mater Med. 2019 Jun 20;30(7):78. doi: 10.1007/s10856-019-6279-x. J Mater Sci Mater Med. 2019. PMID: 31222566 - Enhanced adhesion and differentiation of human mesenchymal stem cell inside apatite-mineralized/poly(dopamine)-coated poly(ε-caprolactone) scaffolds by stereolithography.
Cheng YL , Chen YW , Wang K , Shie MY . Cheng YL , et al. J Mater Chem B. 2016 Oct 14;4(38):6307-6315. doi: 10.1039/c6tb01377e. Epub 2016 Sep 12. J Mater Chem B. 2016. PMID: 32263532 - Emerging Biomedical and Clinical Applications of 3D-Printed Poly(Lactic Acid)-Based Devices and Delivery Systems.
Barcena AJR, Ravi P, Kundu S, Tappa K. Barcena AJR, et al. Bioengineering (Basel). 2024 Jul 11;11(7):705. doi: 10.3390/bioengineering11070705. Bioengineering (Basel). 2024. PMID: 39061787 Free PMC article. Review.
Cited by
- Polydopamine-Based Biomaterials in Orthopedic Therapeutics: Properties, Applications, and Future Perspectives.
Zhang M, Mi M, Hu Z, Li L, Chen Z, Gao X, Liu D, Xu B, Liu Y. Zhang M, et al. Drug Des Devel Ther. 2024 Aug 26;18:3765-3790. doi: 10.2147/DDDT.S473007. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39219693 Free PMC article. Review. - Bombyx mori Silk Fibroin Scaffolds with Antheraea pernyi Silk Fibroin Micro/Nano Fibers for Promoting EA. hy926 Cell Proliferation.
Chen Y, Yang W, Wang W, Zhang M, Li M. Chen Y, et al. Materials (Basel). 2017 Oct 3;10(10):1153. doi: 10.3390/ma10101153. Materials (Basel). 2017. PMID: 28972553 Free PMC article. - Surface Modification of Calcium Silicate via Mussel-Inspired Polydopamine and Effective Adsorption of Extracellular Matrix to Promote Osteogenesis Differentiation for Bone Tissue Engineering.
Kao CT, Chen YJ, Ng HY, Lee AK, Huang TH, Lin TF, Hsu TT. Kao CT, et al. Materials (Basel). 2018 Sep 9;11(9):1664. doi: 10.3390/ma11091664. Materials (Basel). 2018. PMID: 30205589 Free PMC article. - MicroRNAs delivery into human cells grown on 3D-printed PLA scaffolds coated with a novel fluorescent PAMAM dendrimer for biomedical applications.
Paolini A, Leoni L, Giannicchi I, Abbaszadeh Z, D'Oria V, Mura F, Dalla Cort A, Masotti A. Paolini A, et al. Sci Rep. 2018 Sep 17;8(1):13888. doi: 10.1038/s41598-018-32258-9. Sci Rep. 2018. PMID: 30224665 Free PMC article. - The Fabrication and Bonding of Thermoplastic Microfluidics: A Review.
Shakeri A, Khan S, Jarad NA, Didar TF. Shakeri A, et al. Materials (Basel). 2022 Sep 18;15(18):6478. doi: 10.3390/ma15186478. Materials (Basel). 2022. PMID: 36143790 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous