An Evaluation of the in vivo Safety of Nonporous Silica Nanoparticles: Ocular Topical Administration versus Oral Administration - PubMed (original) (raw)
An Evaluation of the in vivo Safety of Nonporous Silica Nanoparticles: Ocular Topical Administration versus Oral Administration
Martha Kim et al. Sci Rep. 2017.
Abstract
Nonporous silica nanoparticles (SiNPs) have potential as promising carriers for ophthalmic drugs. However, the in vivo safety of ocular topical SiNPs remains unclear. This study investigated the in vivo safety of oral and ocular topical applications of 100 nm-sized SiNPs in Sprague-Dawley rats. The rats were divided into the following four groups: low-dose oral administration (total 100 mg/kg of SiNPs mixed with food for one week), high-dose oral administration (total 1000 mg/kg of SiNPs mixed with food for one week), ocular topical administration (10 mg/ml concentration, one drop, applied to the right eyes four times a day for one month), or a negative control (no SiNP treatment). The rats were observed for 12 weeks to investigate any signs of general or ocular toxicity. During the observation period, no differences were observed in the body weights, food and water intakes, behaviors and abnormal symptoms of the four groups. No animal deaths occurred. After 12 weeks, hematologic, blood biochemical parameters and ophthalmic examinations revealed no abnormal findings in any of the animals. The lack of toxicity of the SiNPs was further verified in autopsy findings of brain, liver, lung, spleen, heart, kidneys, intestine, eyeballs, and ovaries or testes.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
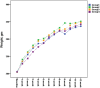
Body weight of the rats following the SiNP applications. There was no statistically significant difference in the body weights of the different groups (Group 1, control; Group 2, oral intake, 100 mg/kg; Group 3, oral intake, 1000 mg/kg; Group 4, topical application, 10 mg/ml) over the 12-week period.
Figure 2
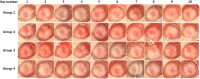
In vivo corneal effects of the SiNP applications. Digital photographs were obtained every week for 12 weeks. Corneal transparency and limbal vessels were normal in all the treated groups (Group 2, oral intake 100 mg/kg; Group 3, oral intake 1000 mg/kg; Group 4, topical application 10 mg/ml) as compared to those of the controls (Group 1).
Figure 3
In vivo imaging of the optic nerve head following the SiNP applications. All the images were obtained 12 weeks after the treatments. There was no evidence of optic nerve damage or vascular changes in any of the treated groups (Group 2, oral intake 100 mg/kg; Group 3, oral intake 1000 mg/kg; Group 4, topical application 10 mg/ml) compared to the controls (Group 1).
Figure 4
Histopathological findings of organs from the rats treated with SiNPs. Kidney, liver, spleen, lung, and eyeball samples were collected 12 weeks after the SiNP applications. Sections were stained with hematoxylin and eosin (×200): No treatment (Group 1, controls); low-dose oral intake (Group 2, 100 mg/kg); high-dose oral intake (Group 3, 1000 mg/kg); topical application (Group 4, 10 mg/ml).
Similar articles
- Toxicity study of silica nanoparticles following 94-day repeated oral administration in Sprague Dawley rats.
Cao X, Xie B, Xu M, Li J, Dai X, Tian Y, Zhang J, Chen Y, Yan L, Zhang B, Shi W, Ren L. Cao X, et al. Naunyn Schmiedebergs Arch Pharmacol. 2025 May;398(5):5661-5676. doi: 10.1007/s00210-024-03639-x. Epub 2024 Nov 27. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39601819 - The Effects of Nonporous Silica Nanoparticles on Cultured Human Keratocytes.
Yim B, Park JH, Jeong H, Hong J, Shin YJ, Chuck RS, Park CY. Yim B, et al. Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):362-371. doi: 10.1167/iovs.16-20603. Invest Ophthalmol Vis Sci. 2017. PMID: 28118663 - Optical imaging of absorption and distribution of RITC-SiO2 nanoparticles after oral administration.
Lee CM, Lee TK, Kim DI, Kim YR, Kim MK, Jeong HJ, Sohn MH, Lim ST. Lee CM, et al. Int J Nanomedicine. 2014 Dec 15;9 Suppl 2(Suppl 2):243-50. doi: 10.2147/IJN.S57938. eCollection 2014. Int J Nanomedicine. 2014. PMID: 25565842 Free PMC article. - Silica nanoparticles: Biomedical applications and toxicity.
Huang Y, Li P, Zhao R, Zhao L, Liu J, Peng S, Fu X, Wang X, Luo R, Wang R, Zhang Z. Huang Y, et al. Biomed Pharmacother. 2022 Jul;151:113053. doi: 10.1016/j.biopha.2022.113053. Epub 2022 May 17. Biomed Pharmacother. 2022. PMID: 35594717 Review. - Functionalized silica nanoparticles: a platform for fluorescence imaging at the cell and small animal levels.
Wang K, He X, Yang X, Shi H. Wang K, et al. Acc Chem Res. 2013 Jul 16;46(7):1367-76. doi: 10.1021/ar3001525. Epub 2013 Mar 14. Acc Chem Res. 2013. PMID: 23489227 Review.
Cited by
- Evaluating the Effects of Chronic Oral Exposure to the Food Additive Silicon Dioxide on Oral Tolerance Induction and Food Sensitivities in Mice.
Lamas B, Martins Breyner N, Malaisé Y, Wulczynski M, Galipeau HJ, Gaultier E, Cartier C, Verdu EF, Houdeau E. Lamas B, et al. Environ Health Perspect. 2024 Feb;132(2):27007. doi: 10.1289/EHP12758. Epub 2024 Feb 21. Environ Health Perspect. 2024. PMID: 38380914 Free PMC article. - High-Capacity Mesoporous Silica Nanocarriers of siRNA for Applications in Retinal Delivery.
Ultimo A, Orzaez M, Santos-Martinez MJ, Martínez-Máñez R, Marcos MD, Sancenón F, Ruiz-Hernández E. Ultimo A, et al. Int J Mol Sci. 2023 Feb 1;24(3):2753. doi: 10.3390/ijms24032753. Int J Mol Sci. 2023. PMID: 36769075 Free PMC article. - Mechanistic Insights into the Antimicrobial Actions of Metallic Nanoparticles and Their Implications for Multidrug Resistance.
Shaikh S, Nazam N, Rizvi SMD, Ahmad K, Baig MH, Lee EJ, Choi I. Shaikh S, et al. Int J Mol Sci. 2019 May 18;20(10):2468. doi: 10.3390/ijms20102468. Int J Mol Sci. 2019. PMID: 31109079 Free PMC article. Review. - Silica Nanoparticles-A Versatile Tool for the Treatment of Bacterial Infections.
Selvarajan V, Obuobi S, Ee PLR. Selvarajan V, et al. Front Chem. 2020 Jul 15;8:602. doi: 10.3389/fchem.2020.00602. eCollection 2020. Front Chem. 2020. PMID: 32760699 Free PMC article. Review. - Safety Assessment of Nanomaterials to Eyes: An Important but Neglected Issue.
Zhu S, Gong L, Li Y, Xu H, Gu Z, Zhao Y. Zhu S, et al. Adv Sci (Weinh). 2019 Jun 20;6(16):1802289. doi: 10.1002/advs.201802289. eCollection 2019 Aug 21. Adv Sci (Weinh). 2019. PMID: 31453052 Free PMC article. Review.
References
- Molokhia SA, Thomas SC, Garff KJ, Mandell KJ, Wirostko BM. Anterior eye segment drug delivery systems: current treatments and future challenges. Journal of ocular pharmacology and therapeutics: the official journal of the Association for Ocular Pharmacology and Therapeutics. 2013;29:92–105. doi: 10.1089/jop.2012.0241. - DOI - PubMed
- Liu, S., Jones, L. & Gu, F. X. Nanomaterials for ocular drug delivery. Macromolecular bioscience12, 608–620, doi:10.1002/mabi.201100419 (2012). - PubMed
- Sharma OP, Patel V, Mehta T. Nanocrystal for ocular drug delivery: hope or hype. Drug delivery and translational research. 2016 - PubMed
- Verma, P. & Ahuja, M. Cubic liquid crystalline nanoparticles: optimization and evaluation for ocular delivery of tropicamide. Drug delivery, 1–12, doi:10.3109/10717544.2016.1143057 (2016). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources