Amyloid β-42 induces neuronal apoptosis by targeting mitochondria - PubMed (original) (raw)
Amyloid β-42 induces neuronal apoptosis by targeting mitochondria
Xiao-Jian Han et al. Mol Med Rep. 2017 Oct.
Abstract
Alzheimer's disease (AD), with a typical pathological hallmark of amyloid‑beta (Aβ)‑containing plaques and neurofibrillary tangles, is one of the most common types of chronic neurodegenerative diseases. Aβ oligomers serve a crucial role in the pathogenesis of AD, and lead to neuronal loss. However, the precise mechanism of Aβ oligomers in AD remains to be elucidated. The present study demonstrated that 10 µM Aβ‑42 activated the caspase signaling pathway, and induced significant apoptosis in primary cultured mouse cerebral cortical neurons. The results of reverse transcription‑quantitative polymerase chain reaction and western blotting demonstrated that Aβ‑42 (10 µM) also significantly upregulated the transcription and expression of the mitochondrial fission protein dynamin‑related protein 1 (Drp1), and downregulated the transcription and expression of mitochondrial fusion proteins, including mitofusin 1/2 (Mfn1/2) and mitochondrial dynamin like GTPase (OPA‑1). Neurons were transfected with pDsRed2‑Mito for mitochondrial imaging, which revealed that 10 µM Aβ‑42 induced mitochondrial fission in cortical neurons. In addition, 2',7'‑dichlorodihydrofluorescein diacetate and tetramethylrhodamine ethyl ester staining indicated that Aβ‑42 increased the reactive oxygen species (ROS) level and reduced mitochondrial membrane potential in neurons. Inhibition of Drp1 activity by Mdivi‑1 efficiently prevented Aβ‑42‑induced ROS production and disruption of mitochondrial membrane potential. Loss of mitochondrial membrane potential may activate PTEN‑induced putative kinase 1 (Pink1), the prominent sensor for mitochondrial damage, and trigger the process of mitophagy to remove the damaged mitochondria. In the present study, western blotting revealed that the levels of autophagy marker microtubule‑associated proteins 1A/1B light chain 3B (LC3B) and Pink1 were upregulated after Aβ‑42 stimulation. In conclusion, these data indicated that Aβ‑42 induces neuronal apoptosis by targeting mitochondria, including promotion of mitochondrial fission, disruption of mitochondrial membrane potential, increasing intracellular ROS level and activation of the process of mitophagy. Therefore, mitochondria may represent a potential therapeutic target for AD in the future.
Figures
Figure 1.
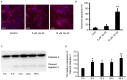
Effect of Aβ-42 at different concentrations on mouse cerebral cortical neurons. Representative fluorescence microscopy images of mouse cerebral cortical neurons (10 days in vitro) exposed to Aβ-42. Scale bar=20 µm. Aβ-42, amyloid β-42.
Figure 2.
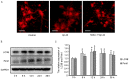
Aβ-42-induced cortical neuronal apoptosis and activated caspase pathway. (A) Representative fluorescence microscopy images and (B) quantification of neuronal apoptosis of mouse cerebral cortical neurons (10 days in vitro) exposed to Aβ-42 and stained with Hoechst 33258 and MAP2. Scale bar=50 µm. (C) Representative western blot images and (D) quantification of caspase 3 protein expression levels. Data are presented as the mean ± standard deviation of at least three independent experiments. *P<0.05 vs. 0 h, **P<0.01 vs. Cont or 0 h. Cont, control; Aβ-42, amyloid β-42.
Figure 3.
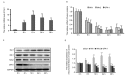
Transcription and expression of mitochondrial fission and fusion proteins after Aβ-42 treatment. mRNA expression levels of (A) Drp1 and (B) Mfn1, Mfn2 and OPA-1. (C) Representative western blot images and (D) quantification of Drp1, Mfn1, Mfn2 and OPN-1 protein expression levels. GAPDH served as an endogenous control. Data are presented as the mean ± standard deviation of at least three independent experiments. *P<0.05, **P<0.01 vs. 0 h. Aβ-42, amyloid β-42; Drp1, dynamin-related protein 1; Mfn, mitofusin; OPA-1, mitochondrial dynamin like GTPase.
Figure 4.
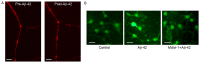
Effect of Aβ-42 on mitochondrial fission and intracellular ROS production. (A) Mitochondrial morphology in neurons pre- and post-treatment with Aβ-42. Scale bar=5 µm. (B) Effect of Aβ-42 on intracellular ROS production. Scale bar=20 µm. ROS, reactive oxygen species; Aβ-42, amyloid β-42.
Figure 5.
Aβ-42 disrupts mitochondrial membrane potential and upregulates expression of LC3B and Pink1. (A) Effect of Aβ-42 on mitochondrial membrane potential in neurons, as assessed by tetramethylrhodamine ethyl ester staining. Scale bar=20 µm. (B) Representative western blot images and (C) quantification of protein expression levels of LC3B and Pink1 after Aβ-42 treatment. GAPDH was used as an endogenous control. Data are presented as the mean ± standard deviation of at least three independent experiments. *P<0.05 vs. 0 h. Aβ-42, amyloid β-42; LC3B, microtubule-associated proteins 1A/1B light chain 3B.
Similar articles
- Aβ-Induced Drp1 phosphorylation through Akt activation promotes excessive mitochondrial fission leading to neuronal apoptosis.
Kim DI, Lee KH, Gabr AA, Choi GE, Kim JS, Ko SH, Han HJ. Kim DI, et al. Biochim Biophys Acta. 2016 Nov;1863(11):2820-2834. doi: 10.1016/j.bbamcr.2016.09.003. Epub 2016 Sep 4. Biochim Biophys Acta. 2016. PMID: 27599716 - Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy.
Xian H, Liou YC. Xian H, et al. Autophagy. 2019 Dec;15(12):2107-2125. doi: 10.1080/15548627.2019.1596494. Epub 2019 Mar 28. Autophagy. 2019. PMID: 30894073 Free PMC article. - Amyloid Beta and Phosphorylated Tau-Induced Defective Autophagy and Mitophagy in Alzheimer's Disease.
Reddy PH, Oliver DM. Reddy PH, et al. Cells. 2019 May 22;8(5):488. doi: 10.3390/cells8050488. Cells. 2019. PMID: 31121890 Free PMC article. Review. - Ethanol-activated CaMKII signaling induces neuronal apoptosis through Drp1-mediated excessive mitochondrial fission and JNK1-dependent NLRP3 inflammasome activation.
Lim JR, Lee HJ, Jung YH, Kim JS, Chae CW, Kim SY, Han HJ. Lim JR, et al. Cell Commun Signal. 2020 Aug 12;18(1):123. doi: 10.1186/s12964-020-00572-3. Cell Commun Signal. 2020. PMID: 32787872 Free PMC article. - Recent Advances in Molecular Pathways and Therapeutic Implications Targeting Mitochondrial Dysfunction for Alzheimer's Disease.
Dhapola R, Sarma P, Medhi B, Prakash A, Reddy DH. Dhapola R, et al. Mol Neurobiol. 2022 Jan;59(1):535-555. doi: 10.1007/s12035-021-02612-6. Epub 2021 Nov 2. Mol Neurobiol. 2022. PMID: 34725778 Review.
Cited by
- Magnetic stirring with iron oxide nanospinners accretes neurotoxic Aβ42 oligomers into phagocytic clearable plaques for Alzheimer's disease treatment.
Sabu A, Huang YC, Sharmila R, Sun CY, Shen MY, Chiu HC. Sabu A, et al. Mater Today Bio. 2024 Aug 27;28:101213. doi: 10.1016/j.mtbio.2024.101213. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39280110 Free PMC article. - Scattering Angle Resolved Optical Coherence Tomography Detects Early Changes in 3xTg Alzheimer's Disease Mouse Model.
Gardner MR, Baruah V, Vargas G, Motamedi M, Milner TE, Rylander HG 3rd. Gardner MR, et al. Transl Vis Sci Technol. 2020 Apr 24;9(5):18. doi: 10.1167/tvst.9.5.18. eCollection 2020 Apr. Transl Vis Sci Technol. 2020. PMID: 32821490 Free PMC article. - Adiponectin: The Potential Regulator and Therapeutic Target of Obesity and Alzheimer's Disease.
Kim JY, Barua S, Jeong YJ, Lee JE. Kim JY, et al. Int J Mol Sci. 2020 Sep 3;21(17):6419. doi: 10.3390/ijms21176419. Int J Mol Sci. 2020. PMID: 32899357 Free PMC article. Review. - Comparative Effects of Alpha- and Gamma-Tocopherol on Mitochondrial Functions in Alzheimer's Disease In Vitro Model.
Pahrudin Arrozi A, Shukri SNS, Wan Ngah WZ, Mohd Yusof YA, Ahmad Damanhuri MH, Jaafar F, Makpol S. Pahrudin Arrozi A, et al. Sci Rep. 2020 Jun 2;10(1):8962. doi: 10.1038/s41598-020-65570-4. Sci Rep. 2020. PMID: 32488024 Free PMC article. - An Analysis of the Neurological and Molecular Alterations Underlying the Pathogenesis of Alzheimer's Disease.
Vidal C, Zhang L. Vidal C, et al. Cells. 2021 Mar 4;10(3):546. doi: 10.3390/cells10030546. Cells. 2021. PMID: 33806317 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous