Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells - PubMed (original) (raw)
Bone regeneration in minipigs by intrafibrillarly-mineralized collagen loaded with autologous periodontal ligament stem cells
Ci Zhang et al. Sci Rep. 2017.
Abstract
Biomimetic intrafibrillarly-mineralized collagen (IMC) is a promising scaffold for bone regeneration because of its structural and functional similarity to natural bone. The objective of this study was to evaluate the bone regeneration potential of IMC loaded with autologous periodontal ligament stem cells (PDLSCs) in large bone defects in minipigs. A macroporous IMC with a bone-like subfibrillar nanostructure was fabricated using a biomimetic bottom-up approach. Non-healing full thickness defects were established on the cranial bone in minipigs, and IMC and hydroxyapatite (HA) scaffolds seeded with autologous PDLSCs were implanted into these defects. Computed tomographic imaging, histology staining, and atomic force microscopy were applied to evaluate to the quantity, micro/nano structures, and mechanical performance of the neo-bone after 12 weeks of implantation. Compared with HA, IMC showed superior regeneration properties characterized by the profuse deposition of new bony structures with a normal architecture and vascularization. Immunohistochemistry showed that the runt-related transcription factor 2 and transcription factor Osterix were highly expressed in the neo-bone formed by IMC. Furthermore, the nanostructure and nanomechanics of the neo-bone formed by IMC were similar to that of natural bone. This study provides strong evidence for the future clinical applications of the IMC-based bone grafts.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Figure 1
Morphological and elemental analysis of different scaffolds. (A) Representative (left) low- and (right) high-magnification SEM images of cross-sections of IMC and HA scaffolds. (B) EDS in different scaffolds, confirming the presence of apatite crystallites within the collagen fibrils in the IMC scaffold. Inset: AFM morphology of IMC showing obvious cross-banding patterns.
Figure 2
Representative SEM images of PDLSCs cultured on different scaffolds. (A) On day 3, PDLSCs attached to all the scaffolds, but only secreted ECM (black arrows) on the IMC scaffold. (B) On day 7, abundant fibrous ECM was secreted in the IMC group and calcified nodules deposited on the cell surface in the HA group.
Figure 3
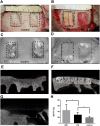
Surgical procedure for producing a non-healing defect and representative CT images of bone regeneration by different scaffolds. (A) A non-healing defect of 2 cm width × 3 cm length × 0.5 cm depth in minipig cranium. (B) The defects were respectively filled with HA and IMC scaffolds seeded with PDLSCs. (C,D) Representative 3-D reconstructed images in the control (C) and experimental groups (D). (E–G) Representative cross-section images of defect area after implantation with IMC (E) and HA (F), and without implants (G) for 12 weeks. (H) Volume analysis of the defect area based on CT results. Groups labeled with star are significantly different (P < 0.05).
Figure 4
HE and Masson’s trichrome staining of defect area after implantation with different scaffolds for 12 weeks. Plenty neo-bone with osteon and vessles was formed by IMC, whereas limited newly-formed bone and lots of residual scaffolds were observed in the HA group. A small amount of newly-formed bone was seen around the defect margin in the control group. B: natural bone; S: scaffold; NB: new bone; DM: defect margin (black dotted line); O: osteon; V: vessel.
Figure 5
Immunohistochemical staining of Runx2, Osx, and TGF-β1 in the defect areas of the two in vivo groups. Runx2, Osx and TGF-β1 were highly expressed in the IMC group, whereas weak or negative staining was observed in the HA group.
Figure 6
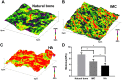
Nanomechanics of bone tissue by AFM. (A–C) Representative 3-D AFM property maps of natural bone (A), and newly-formed bone in the IMC group (B) and HA group (C). (D) Quantitative analysis of Young’s modulus in different groups. Groups labeled with star are significantly different (*P < 0.05).
Similar articles
- Human Periodontal Ligament Stem Cells Transplanted with Nanohydroxyapatite/Chitosan/Gelatin 3D Porous Scaffolds Promote Jaw Bone Regeneration in Swine.
Zhao Q, Li G, Wang T, Jin Y, Lu W, Ji J. Zhao Q, et al. Stem Cells Dev. 2021 May 15;30(10):548-559. doi: 10.1089/scd.2020.0204. Epub 2021 Apr 27. Stem Cells Dev. 2021. PMID: 33736461 - Surface Chemistry of Nanoscale Mineralized Collagen Regulates Periodontal Ligament Stem Cell Fate.
Fu Y, Liu S, Cui SJ, Kou XX, Wang XD, Liu XM, Sun Y, Wang GN, Liu Y, Zhou YH. Fu Y, et al. ACS Appl Mater Interfaces. 2016 Jun 29;8(25):15958-66. doi: 10.1021/acsami.6b04951. Epub 2016 Jun 17. ACS Appl Mater Interfaces. 2016. PMID: 27280804 - Mineralized Collagen Regulates Macrophage Polarization During Bone Regeneration.
Sun Y, Liu S, Fu Y, Kou XX, He DQ, Wang GN, Fu CC, Liu Y, Zhou YH. Sun Y, et al. J Biomed Nanotechnol. 2016 Nov;12(11):2029-40. doi: 10.1166/jbn.2016.2296. J Biomed Nanotechnol. 2016. PMID: 29364617 - Bioinspired Collagen-Apatite Nanocomposites for Bone Regeneration.
Liu S, Sun Y, Fu Y, Chang D, Fu C, Wang G, Liu Y, Tay FR, Zhou Y. Liu S, et al. J Endod. 2016 Aug;42(8):1226-32. doi: 10.1016/j.joen.2016.04.027. Epub 2016 Jul 2. J Endod. 2016. PMID: 27377439 - Bioinspired Collagen Scaffolds in Cranial Bone Regeneration: From Bedside to Bench.
Lee JC, Volpicelli EJ. Lee JC, et al. Adv Healthc Mater. 2017 Sep;6(17):10.1002/adhm.201700232. doi: 10.1002/adhm.201700232. Epub 2017 Jun 6. Adv Healthc Mater. 2017. PMID: 28585295 Free PMC article. Review.
Cited by
- Clinical Applications of Cell-Scaffold Constructs for Bone Regeneration Therapy.
Venkataiah VS, Yahata Y, Kitagawa A, Inagaki M, Kakiuchi Y, Nakano M, Suzuki S, Handa K, Saito M. Venkataiah VS, et al. Cells. 2021 Oct 8;10(10):2687. doi: 10.3390/cells10102687. Cells. 2021. PMID: 34685667 Free PMC article. Review. - Nanomaterials Modulating the Fate of Dental-Derived Mesenchymal Stem Cells Involved in Oral Tissue Reconstruction: A Systematic Review.
Li X, Wang Y, Huang D, Jiang Z, He Z, Luo M, Lei J, Xiao Y. Li X, et al. Int J Nanomedicine. 2023 Sep 21;18:5377-5406. doi: 10.2147/IJN.S418675. eCollection 2023. Int J Nanomedicine. 2023. PMID: 37753067 Free PMC article. Review. - Potential of Bone-Marrow-Derived Mesenchymal Stem Cells for Maxillofacial and Periodontal Regeneration: A Narrative Review.
Khorasani HR, Sanchouli M, Mehrani J, Sabour D. Khorasani HR, et al. Int J Dent. 2021 Nov 9;2021:4759492. doi: 10.1155/2021/4759492. eCollection 2021. Int J Dent. 2021. PMID: 34795761 Free PMC article. Review. - Functionalization of biomimetic mineralized collagen for bone tissue engineering.
Zhu X, Wang C, Bai H, Zhang J, Wang Z, Li Z, Zhao X, Wang J, Liu H. Zhu X, et al. Mater Today Bio. 2023 May 6;20:100660. doi: 10.1016/j.mtbio.2023.100660. eCollection 2023 Jun. Mater Today Bio. 2023. PMID: 37214545 Free PMC article. Review. - Potential of Oral Cavity Stem Cells for Bone Regeneration: A Scoping Review.
Alarcón-Apablaza J, Prieto R, Rojas M, Fuentes R. Alarcón-Apablaza J, et al. Cells. 2023 May 15;12(10):1392. doi: 10.3390/cells12101392. Cells. 2023. PMID: 37408226 Free PMC article. Review.
References
- Betz RR. Limitations of autograft and allograft: New synthetic solutions. Orthopedics. 2002;25:S561–S570. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources