Multi-Target Screening and Experimental Validation of Natural Products from Selaginella Plants against Alzheimer's Disease - PubMed (original) (raw)
Multi-Target Screening and Experimental Validation of Natural Products from Selaginella Plants against Alzheimer's Disease
Yin-Hua Deng et al. Front Pharmacol. 2017.
Abstract
Alzheimer's disease (AD) is a progressive and irreversible neurodegenerative disorder which is considered to be the most common cause of dementia. It has a greater impact not only on the learning and memory disturbances but also on social and economy. Currently, there are mainly single-target drugs for AD treatment but the complexity and multiple etiologies of AD make them difficult to obtain desirable therapeutic effects. Therefore, the choice of multi-target drugs will be a potential effective strategy inAD treatment. To find multi-target active ingredients for AD treatment from Selaginella plants, we firstly explored the behaviors effects on AD mice of total extracts (TE) from Selaginella doederleinii on by Morris water maze test and found that TE has a remarkable improvement on learning and memory function for AD mice. And then, multi-target SAR models associated with AD-related proteins were built based on Random Forest (RF) and different descriptors to preliminarily screen potential active ingredients from Selaginella. Considering the prediction outputs and the quantity of existing compounds in our laboratory, 13 compounds were chosen to carry out the in vitro enzyme inhibitory experiments and 4 compounds with BACE1/MAO-B dual inhibitory activity were determined. Finally, the molecular docking was applied to verify the prediction results and enzyme inhibitory experiments. Based on these study and validation processes, we explored a new strategy to improve the efficiency of active ingredients screening based on trace amount of natural product and numbers of targets and found some multi-target compounds with biological activity for the development of novel drugs for AD treatment.
Keywords: Alzheimer; BACE1; MAO-B; Selaginella plants; multi-target SAR; multi-target screening.
Figures
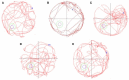
Figure 1
The spatial learning and memory ability of AD mice tested by Morris water maze [(A) NCG: normal control group; (B) MCG: model control group; (C) LDG: low dose group; (D) MDG: middle dose group; (E) HDG: high dose group]. This figure shows that TE has a remarkable improvement on learning and memory function for AD mice which mainly lies in the increased distance and this functional improvement is dose-dependent.
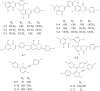
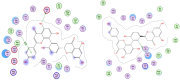
Figure 2
The chemical structures of 13 compounds that with inhibitory activity after multi-target SAR model prediction. Among them, eight are biflavones and the left five are selaginellins.
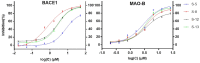
Figure 3
Verification of BACE1 and MAO-B Inhibition. This figure shows that all these four compounds (S-5, S-8, S-12, S-13) have good inhibitory activity in the in vitro validation test.
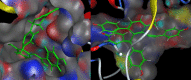
Figure 4
The docking results of S-8 bounding to BACE1 (left, PDB ID: 1TQF) and MAO-B (right, PDB ID: 2V5Z). The structure of S-8 is rendered green and the docking pocket surface was adjected to a suitable transparency.
Figure 5
The ligand interaction diagram of S-8 bounding to BACE1 (left, PDB ID: 1TQF) and MAO-B (right, PDB ID: 2V5Z). It is a 2D diagram of the original ligand and a schematic representation of the binding site residues, with the important interactions between ligand and binding site shown. For BACE1, the main binding force is the hydrogen bond force and pi-bond force with ASP (232A) and THR (231A); for MAO-B, the main binding force is the hydrogen bond force and pi-bond force with CYS (397A) and GLY (13A).
Similar articles
- Identifying natural compounds as multi-target-directed ligands against Alzheimer's disease: an in silico approach.
Ambure P, Bhat J, Puzyn T, Roy K. Ambure P, et al. J Biomol Struct Dyn. 2019 Mar;37(5):1282-1306. doi: 10.1080/07391102.2018.1456975. Epub 2018 Apr 23. J Biomol Struct Dyn. 2019. PMID: 29578387 - Features and outcomes of drugs for combination therapy as multi-targets strategy to combat Alzheimer's disease.
Sahoo AK, Dandapat J, Dash UC, Kanhar S. Sahoo AK, et al. J Ethnopharmacol. 2018 Apr 6;215:42-73. doi: 10.1016/j.jep.2017.12.015. Epub 2017 Dec 14. J Ethnopharmacol. 2018. PMID: 29248451 Review. - A strategy to find novel candidate anti-Alzheimer's disease drugs by constructing interaction networks between drug targets and natural compounds in medical plants.
Chen BW, Li WX, Wang GH, Li GH, Liu JQ, Zheng JJ, Wang Q, Li HJ, Dai SX, Huang JF. Chen BW, et al. PeerJ. 2018 May 11;6:e4756. doi: 10.7717/peerj.4756. eCollection 2018. PeerJ. 2018. PMID: 29770277 Free PMC article. - Identification of Multi-Target Anti-AD Chemical Constituents From Traditional Chinese Medicine Formulae by Integrating Virtual Screening and In Vitro Validation.
Zhang B, Zhao J, Wang Z, Guo P, Liu A, Du G. Zhang B, et al. Front Pharmacol. 2021 Jul 16;12:709607. doi: 10.3389/fphar.2021.709607. eCollection 2021. Front Pharmacol. 2021. PMID: 34335272 Free PMC article. - Multi-target-directed ligands in Alzheimer's disease treatment.
Bajda M, Guzior N, Ignasik M, Malawska B. Bajda M, et al. Curr Med Chem. 2011;18(32):4949-75. doi: 10.2174/092986711797535245. Curr Med Chem. 2011. PMID: 22050745 Review.
Cited by
- Automated machine learning approach for developing a quantitative structure-activity relationship model for cardiac steroid inhibition of Na+/K+-ATPase.
Takada Y, Kaneko K. Takada Y, et al. Pharmacol Rep. 2023 Aug;75(4):1017-1025. doi: 10.1007/s43440-023-00508-x. Epub 2023 Jun 24. Pharmacol Rep. 2023. PMID: 37354314 - Embelin, a Potent Molecule for Alzheimer's Disease: A Proof of Concept From Blood-Brain Barrier Permeability, Acetylcholinesterase Inhibition and Molecular Docking Studies.
Bhuvanendran S, Hanapi NA, Ahemad N, Othman I, Yusof SR, Shaikh MF. Bhuvanendran S, et al. Front Neurosci. 2019 May 16;13:495. doi: 10.3389/fnins.2019.00495. eCollection 2019. Front Neurosci. 2019. PMID: 31156375 Free PMC article. - Trilobatin Protects Against Aβ25-35-Induced Hippocampal HT22 Cells Apoptosis Through Mediating ROS/p38/Caspase 3-Dependent Pathway.
Chen N, Wang J, He Y, Xu Y, Zhang Y, Gong Q, Yu C, Gao J. Chen N, et al. Front Pharmacol. 2020 May 19;11:584. doi: 10.3389/fphar.2020.00584. eCollection 2020. Front Pharmacol. 2020. PMID: 32508629 Free PMC article. - Molecular docking analysis of flavonoids with AChE and BACE-1.
Viswanathan S, Arumugam T, Subramanian K, Sivaraj R, Ramesh V, Vasanthi AHR. Viswanathan S, et al. Bioinformation. 2024 Feb 29;20(2):103-109. doi: 10.6026/973206300200103. eCollection 2024. Bioinformation. 2024. PMID: 38497082 Free PMC article. - Screening of antibacterial compounds with novel structure from the FDA approved drugs using machine learning methods.
Li WX, Tong X, Yang PP, Zheng Y, Liang JH, Li GH, Liu D, Guan DG, Dai SX. Li WX, et al. Aging (Albany NY). 2022 Feb 12;14(3):1448-1472. doi: 10.18632/aging.203887. Epub 2022 Feb 12. Aging (Albany NY). 2022. PMID: 35150482 Free PMC article.
References
- Breiman L. (2001). Random forests. Mach. Learn. 45, 5–32. 10.1023/A:1010933404324 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous