New perspectives for targeting RAF kinase in human cancer - PubMed (original) (raw)
Review
. 2017 Nov;17(11):676-691.
doi: 10.1038/nrc.2017.79. Epub 2017 Oct 6.
Affiliations
- PMID: 28984291
- PMCID: PMC6000833
- DOI: 10.1038/nrc.2017.79
Review
New perspectives for targeting RAF kinase in human cancer
Zoi Karoulia et al. Nat Rev Cancer. 2017 Nov.
Abstract
The discovery that a subset of human tumours is dependent on mutationally deregulated BRAF kinase intensified the development of RAF inhibitors to be used as potential therapeutics. The US Food and Drug Administration (FDA)-approved second-generation RAF inhibitors vemurafenib and dabrafenib have elicited remarkable responses and improved survival of patients with BRAF-V600E/K melanoma, but their effectiveness is limited by resistance. Beyond melanoma, current clinical RAF inhibitors show modest efficacy when used for colorectal and thyroid BRAF-V600E tumours or for tumours harbouring BRAF alterations other than the V600 mutation. Accumulated experimental and clinical evidence indicates that the complex biochemical mechanisms of RAF kinase signalling account both for the effectiveness of RAF inhibitors and for the various mechanisms of tumour resistance to them. Recently, a number of next-generation RAF inhibitors, with diverse structural and biochemical properties, have entered preclinical and clinical development. In this Review, we discuss the current understanding of RAF kinase regulation, mechanisms of inhibitor action and related clinical resistance to these drugs. The recent elucidation of critical structural and biochemical aspects of RAF inhibitor action, combined with the availability of a number of structurally diverse RAF inhibitors currently in preclinical and clinical development, will enable the design of more effective RAF inhibitors and RAF-inhibitor-based therapeutic strategies, tailored to different clinical contexts.
Conflict of interest statement
Competing interests statement
The authors declare no competing interests.
Figures
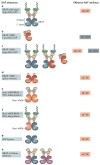
Figure 1. RAF activation
a ∣ Schematic showing canonical RAF activation. In conditions of low RAS-GTP, RAF is monomeric and inactive in the cytosol due to intramolecular interaction between the N-terminal domain (NTD; regulatory) and the C-terminal (kinase) domain (1). Upregulation of RAS-GTP promotes the formation of the RAF–RAS-GTP complex in the membrane due to the high affinity of RAS-GTP for the RAS-binding domain (RBD) present in the NTD of RAF (2). This step is followed by activating phosphorylation and dimerization for full RAF activation (3). αC-helix indicated by the red wavy line in the kinase domain. b ∣ Ribbon representation of one protomer of the active BRAF dimer (Protein Data Bank (PDB) ID: 4MNE). The kinase domain consists of N and C terminal lobes linked through a short flexible hinge. c ∣ Ribbon representation of the inactive and monomeric BRAF kinase domain (PDB ID: 4RZV). In parts b and c, note the different positions of the αC-helix (in green) and of the activation segment (AS, magenta), which highlight the movement of the αC-helix from the OUT to the IN position and the unfolding of the AS upon RAF activation. The R506 residue side chain in the IN (active) and the OUT (inactive) positions and the conformation of the glycine-rich loop (G-loop) and DFG motif in the active and inactive form are also depicted.
Figure 2. Mechanism of action of RAF inhibitors
a ∣ Structural features of representative RAF inhibitors bound to a BRAF dimer. The three structural types of RAF inhibitor bound to BRAF are shown as ribbon representations: Vemurafenib (VEM), a type I1/2 inhibitor (αC-OUT/DFG-IN), AZ628 (AZ), a type II inhibitor (αC-IN/DFG-OUT), and SB590885 (SB), a type I inhibitor (αC-IN/DFG-IN). Vemurafenib and other type I1/2 inhibitors stabilize the αC-helix (red) of the first BRAF protomer to the OUT position and the αC-helix (green) of the second BRAF protomer to the IN position (Protein Data Bank (PDB) ID: 3OG7). AZ628 and other type II inhibitors stabilize the αC-helix of the first BRAF protomer (red) and the αC-helix of the second BRAF protomer (green) to the IN position (PDB ID: 4RZW). SB590885 and other type I inhibitors stabilize both the αC-helix of the first BRAF protomer (red) and the αC-helix of the second BRAF protomer (green) to the IN position (PDB ID: 2FB8). AZ628 and other type II inhibitors induce the DFG motif to an OUT conformation, while other inhibitors of the type I and I1/2 groups maintain the DFG in an IN conformation. b ∣ Schematic showing the αC-OUT RAF inhibitor-induced paradoxical RAF pathway activation and negative allostery. Most αC-OUT RAF inhibitors stabilize the αC-helix (indicated by the red wavy line in the kinase domain) in the overall OUT position (1). However, the C-terminal αC-helix residue R506 is displaced into the IN position, which promotes the interaction of RAF with RAS-GTP (2). Subsequently, RAF is primed through phosphorylation and dimerization. RAF inhibitor binding to one protomer and stabilization of the αC-helix in the OUT position sterically prevents inhibitor binding to the second monomer (negative allostery), leading to paradoxical pathway activity (3). c ∣ Schematic showing αC-IN RAF inhibitor-induced RAF activation and effective dimer inhibition. By stabilizing the αC-helix in the IN position (1), αC-IN RAF inhibitors promote the formation of the RAF–RAS–GTP complex (2). This is followed by RAF phosphorylation and dimerization. Due to low negative allostery, RAF inhibitors belonging to this group effectively bind and inhibit both protomers in the RAF dimer (3). INH, inhibitor; NTD, N-terminal domain.
Figure 3. Categories of RAF alterations found in tumours and the types of RAF inhibitor predicted to be effective against them
a ∣ In the cellular context of RAS-activated wild-type (WT) BRAF, BRAF, CRAF and ARAF form active homodimers and heterodimers that are resistant to αC-OUT RAF inhibitors. αC-IN RAF inhibitors are predicted to potently inhibit RAF dimers, although their effectiveness will be limited due to concurrent formation of active RAF dimers by the inhibitor. b ∣ Mutant BRAF-V600 proteins in the absence of RAS-GTP signal as a monomer and are potently inhibited by either αC-OUT or αC-IN inhibitors. In this context, αC-OUT inhibitors are predicted to be more effective treatments due to their wide therapeutic window. c ∣ In the context of tumours expressing BRAF-V600 in the presence of active RAS, ERK is activated by both BRAF-V600E monomers and RAF dimers (homodimers and heterodimers of BRAF-V600E, WT BRAF, CRAF and ARAF). A combination of a αC-OUT inhibitor with a αC-IN RAF inhibitor is predicted to result in effective RAF inhibition in the tumour while retaining a wide therapeutic window. d ∣ A BRAF-V600E splice variant that lacks part of the N-terminal domain and constitutively dimerizes has been identified in tumours that have developed clinical resistance to αC-OUT RAF inhibitors. In this context, ERK is activated by BRAF-V600E homodimers, and αC-IN RAF inhibitors may be an effective therapeutic option. e ∣ For tumours expressing mutant BRAF proteins other than BRAF-V600 that signal as RAS-independent dimers, αC-IN RAF inhibitors may be an effective therapeutic option. f ∣ For tumours expressing mutant BRAF proteins other than V600 that require RAS activity, αC-IN RAF inhibitors may be an effective therapeutic option. g ∣ Fusion transcripts of various genes with the catalytic domain of either WT BRAF or, in some cases, CRAF, lack the N-terminal domain that prevents RAF dimerization, and their protein products thus signal as RAS-independent RAF dimers. For patients whose tumours depend on such fusion proteins, αC-IN RAF inhibitors may be an effective therapeutic option. h ∣ In-frame deletions in BRAF that stabilize the αC-helix in the active (IN) position have been found in tumours. For patients with such tumours, αC-IN RAF inhibitors may be an effective therapeutic option. C-lobe, C-terminal lobe; N, N-terminal domain; N-lobe, N-terminal lobe; Del, in-frame deletion; αC-IN, αC-helix-IN RAF inhibitor; αC-OUT, αC-helix-OUT RAF inhibitor.
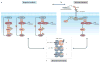
Figure 4. The interplay of mechanisms of adaptive response to RAF inhibitors
a ∣ Relief of negative feedback. At steady state, hyperactivated ERK downstream of mutant BRAF suppresses RAS due to negative feedback. Addition of a RAF inhibitor results in relief of negative feedback and RAS activation, which in turn promotes increased flux through ERK signalling and RAF dimerization,,. Activation indicated by a green halo around the protein. b ∣ Pathway reactivation due to paracrine signalling by components of the tumour microenvironment (TME). Stromal cells in the TME release growth factors (exemplified here by hepatocyte growth factor (HGF)) and cytokines, which promote resistance to RAF inhibitors by activating parallel survival pathways, such as PI3K signalling, or by activating RAS-. c ∣ Structural constraints. RAF dimerization promotes an adaptive response to αC-OUT RAF inhibitors due to negative allostery for inhibitor binding to the second protomer of a RAF dimer when the first is occupied by inhibitor. αC-IN RAF inhibitors are predicted to overcome this resistance mechanism by binding with similar affinity to both protomers of the RAF dimer. All of these mechanisms of adaptive response are interconnected and converge upon structural constraints for inhibitor binding. Relief of negative feedback promotes upstream receptor tyrosine kinase activation, which may be further enhanced by growth factors and cytokines released in the TME. Both mechanisms promote increased flux in the pathway and levels of active RAF molecules. They also promote RAF dimerization, which prevents effective RAF inhibition by current clinical αC-OUT RAF inhibitors due to negative allostery. GAB1, growth factor receptor-bound protein 2-associated binding protein 1; GRB2, growth factor receptor-bound protein 2.
Similar articles
- Current Insights of BRAF Inhibitors in Cancer.
Agianian B, Gavathiotis E. Agianian B, et al. J Med Chem. 2018 Jul 26;61(14):5775-5793. doi: 10.1021/acs.jmedchem.7b01306. Epub 2018 Mar 6. J Med Chem. 2018. PMID: 29461827 Review. - Stamping out RAF and MEK1/2 to inhibit the ERK1/2 pathway: an emerging threat to anticancer therapy.
Mandal R, Becker S, Strebhardt K. Mandal R, et al. Oncogene. 2016 May 19;35(20):2547-61. doi: 10.1038/onc.2015.329. Epub 2015 Sep 14. Oncogene. 2016. PMID: 26364606 Review. - Antitumor activity of the selective pan-RAF inhibitor TAK-632 in BRAF inhibitor-resistant melanoma.
Nakamura A, Arita T, Tsuchiya S, Donelan J, Chouitar J, Carideo E, Galvin K, Okaniwa M, Ishikawa T, Yoshida S. Nakamura A, et al. Cancer Res. 2013 Dec 1;73(23):7043-55. doi: 10.1158/0008-5472.CAN-13-1825. Epub 2013 Oct 11. Cancer Res. 2013. PMID: 24121489 - Phase I/II RAF kinase inhibitors in cancer therapy.
Turajlic S, Ali Z, Yousaf N, Larkin J. Turajlic S, et al. Expert Opin Investig Drugs. 2013 Jun;22(6):739-49. doi: 10.1517/13543784.2013.797964. Epub 2013 May 6. Expert Opin Investig Drugs. 2013. PMID: 23642225 Review. - Targeting the Raf kinases in human cancer: the Raf dimer dilemma.
Durrant DE, Morrison DK. Durrant DE, et al. Br J Cancer. 2018 Jan;118(1):3-8. doi: 10.1038/bjc.2017.399. Epub 2017 Dec 14. Br J Cancer. 2018. PMID: 29235562 Free PMC article. Review.
Cited by
- Activation Loop Dynamics Are Coupled to Core Motions in Extracellular Signal-Regulated Kinase-2.
Iverson DB, Xiao Y, Jones DN, Eisenmesser EZ, Ahn NG. Iverson DB, et al. Biochemistry. 2020 Jul 28;59(29):2698-2706. doi: 10.1021/acs.biochem.0c00485. Epub 2020 Jul 15. Biochemistry. 2020. PMID: 32643366 Free PMC article. - Atypical BRAF and NRAS Mutations in Mucosal Melanoma.
Dumaz N, Jouenne F, Delyon J, Mourah S, Bensussan A, Lebbé C. Dumaz N, et al. Cancers (Basel). 2019 Aug 8;11(8):1133. doi: 10.3390/cancers11081133. Cancers (Basel). 2019. PMID: 31398831 Free PMC article. Review. - Network indirect comparison of 3 BRAF + MEK inhibitors for the treatment of advanced BRAF mutated melanoma.
Consoli F, Bersanelli M, Perego G, Grisanti S, Merelli B, Berruti A, Petrelli F. Consoli F, et al. Clin Transl Oncol. 2020 Jun;22(6):900-907. doi: 10.1007/s12094-019-02207-7. Epub 2019 Sep 25. Clin Transl Oncol. 2020. PMID: 31555967 - Intermittent treatment of BRAFV600E melanoma cells delays resistance by adaptive resensitization to drug rechallenge.
Kavran AJ, Stuart SA, Hayashi KR, Basken JM, Brandhuber BJ, Ahn NG. Kavran AJ, et al. Proc Natl Acad Sci U S A. 2022 Mar 22;119(12):e2113535119. doi: 10.1073/pnas.2113535119. Epub 2022 Mar 15. Proc Natl Acad Sci U S A. 2022. PMID: 35290123 Free PMC article. - Development of Allosteric BRAF Peptide Inhibitors Targeting the Dimer Interface of BRAF.
Gunderwala AY, Nimbvikar AA, Cope NJ, Li Z, Wang Z. Gunderwala AY, et al. ACS Chem Biol. 2019 Jul 19;14(7):1471-1480. doi: 10.1021/acschembio.9b00191. Epub 2019 Jun 17. ACS Chem Biol. 2019. PMID: 31243962 Free PMC article.
References
- Lavoie H, Therrien M. Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol. 2015;16:281–298. - PubMed
- Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. - PubMed
- Wellbrock C, Karasarides M, Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. - PubMed
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous