A Metabolomic Signature of Acute Caloric Restriction - PubMed (original) (raw)
A Metabolomic Signature of Acute Caloric Restriction
Tinh-Hai Collet et al. J Clin Endocrinol Metab. 2017.
Abstract
Context: The experimental paradigm of acute caloric restriction (CR) followed by refeeding (RF) can be used to study the homeostatic mechanisms that regulate energy homeostasis, which are relevant to understanding the adaptive response to weight loss.
Objective: Metabolomics, the measurement of hundreds of small molecule metabolites, their precursors, derivatives, and degradation products, has emerged as a useful tool for the study of physiology and disease and was used here to study the metabolic response to acute CR.
Participants, design, and setting: We used four ultra high-performance liquid chromatography-tandem mass spectrometry methods to characterize changes in carbohydrates, lipids, amino acids, and steroids in eight normal weight men at baseline, after 48 hours of CR (10% of energy requirements) and after 48 hours of ad libitum RF in a tightly controlled environment.
Results: We identified a distinct metabolomic signature associated with acute CR characterized by the expected switch from carbohydrate to fat utilization with increased lipolysis and β-fatty acid oxidation. We found an increase in ω-fatty acid oxidation and levels of endocannabinoids, which are known to promote food intake. These changes were reversed with RF. Several plasmalogen phosphatidylethanolamines (endogenous antioxidants) significantly decreased with CR (all P ≤ 0.0007). Additionally, acute CR was associated with an increase in the branched chain amino acids (all P ≤ 1.4 × 10-7) and dehydroepiandrosterone sulfate (P = 0.0006).
Conclusions: We identified a distinct metabolomic signature associated with acute CR. Further studies are needed to characterize the mechanisms that mediate these changes and their potential contribution to the adaptive response to dietary restriction.
Copyright © 2017 Endocrine Society
Figures
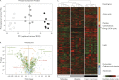
Figure 1.
Metabolomic signature of CR. Seven hundred seventy metabolites were measured in eight subjects at three time points: baseline (gray), after CR (black), and upon RF (white). (A) The PCA; principal component 1 (PC1) captured 38.5% of the variance of the data set and discriminated well between the three study conditions, whereas component 2 (PC2) covered 9.4% of the variance. (B and C) Changes in metabolite categories: amino acids (dark orange), peptides (light orange), carbohydrates (red), energy and the tricarboxylic acid (TCA) cycle (pink), lipids (dark green), nucleotides (light green), cofactors and vitamins (dark blue), and xenobiotics (light blue). (B) A volcano plot of the statistical significance as indicated by P values (_y_-axis) associated with fold change in each metabolite (_x_-axis); baseline to CR (filled circles); CR to RF (open circles). A large percentage of metabolites significantly increased (38%; n = 295) or decreased (39%; n = 300) upon CR. (C) A heatmap derived from hierarchical clustering of the metabolomic data. Clustering was performed using complete linkage and Euclidean distance, where each sample is a vector with all of the metabolite values. The color scale correlates with relative metabolite abundance across the samples: the black indicates median value, red an elevation above the median, and green a decrease below the median.
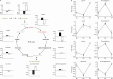
Figure 2.
Glycolysis and gluconeogenesis. (A) Statistically significant increases (red) and decreases (green) in metabolites involved in glycolysis and the TCA cycle with CR (black bars) and upon RF (white bars). Fold changes in glucose, pyruvate, and TCA cycle components are shown (_y_-axis). Some metabolites were not measured in this assay (gray). Statistical significance is presented as follows: + for P values between 0.001 and 0.05; * for P values ≤ 0.001; ** for P values ≤ 0.001 and q values ≤ 0.001. (B) Fold changes in glucogenic amino acids and their _N_-acetyl-derivatives during the study. Plots show mean ± SEM of the eight subjects at baseline (BL; gray), CR (black), and RF (white).
Figure 3.
Fatty acid oxidation and lipolysis. (A) Metabolites involved in lipolysis and fatty acid oxidation that significantly increased (red) or decreased (green) with CR. Some metabolites were not measured in this assay (gray). (B–D) Fold changes in (B) monoacylglycerols (MAG), (C) medium and long-chain fatty acids, and (D) acylcarnitines (CAR) with CR (black) and RF (white). Lipid species are annotated using the following convention: lipid class [number of carbon atoms]:[number of double bonds], [position of double bond(s) if known]. In addition, for MAG, sn1 or sn2 indicate the esterification position in the glycerol backbone, which links the acyl group. Some fatty acids are also designated by abbreviations, such as arachidonic acid (AA), docosahexaenoate (DHA), docosapentaenoate (DPA), and eicosapentaenoate (EPA). (E) Fold changes in ketone bodies between baseline (BL), CR, and RF; mean ± SEM. (F) Fold changes with CR (black) and RF (white) in other lipid species involved in fatty acid oxidation. Statistical significance is presented as follows: + for P values between 0.001 and 0.05; * for P values ≤ 0.001; ** for P values ≤ 0.001 and q values ≤ 0.001.
Figure 4.
Phospholipids and other lipid mediators. Fold changes in phospholipids and other lipid mediators with CR (black) and RF (white): (A) glycerophospholipids, (B) plasmalogens, (C) lysophospholipids, and (D) sphingomyelins. The acyl group(s) are specified using the following convention: [number of carbon atoms]:[number of double bonds], [position of double bond(s) if known]; or the total numbers if unknown. In addition, for LPC and LPE, sn1 or sn2 indicate the esterification position in the glycerol backbone, which links the acyl group. PC(18:0/20:3)a and PC(18:0/20:3)b are structurally similar but differ by the position of the double bonds. The precise structure of PC (38:5)a and PC(38:5)b could not be specified. Statistical significance is presented as follows: + for P values between 0.001 and 0.05; * for P values ≤ 0.001; ** for P values ≤ 0.001 and q values ≤ 0.001. (E) Fold changes in endocannabinoids between baseline (BL), CR, and RF; mean ± SEM. LPC, lysophosphatidylcholine; LPE, lysophosphatidylethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PPC, plasmalogen phosphatidylcholine; PPE, plasmalogen phosphatidylethanolamine; SM, sphingomyelin.
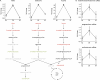
Figure 5.
Amino acids and steroid hormones. (A) Fold changes in the BCAAs valine, isoleucine, and leucine between baseline (BL), CR, and RF; mean ± SEM. Steps in BCAA catabolism are shown; metabolites that significantly increased (red) or decreased (green) with CR are indicated. Some metabolites were not measured in this assay (gray). (B) Fold changes in a subset of steroid hormones between conditions; mean ± SEM.
Similar articles
- Leptin-Mediated Changes in the Human Metabolome.
Lawler K, Huang-Doran I, Sonoyama T, Collet TH, Keogh JM, Henning E, O'Rahilly S, Bottolo L, Farooqi IS. Lawler K, et al. J Clin Endocrinol Metab. 2020 Aug 1;105(8):2541-52. doi: 10.1210/clinem/dgaa251. J Clin Endocrinol Metab. 2020. PMID: 32392278 Free PMC article. Clinical Trial. - The Effects of Graded Levels of Calorie Restriction: XIV. Global Metabolomics Screen Reveals Brown Adipose Tissue Changes in Amino Acids, Catecholamines, and Antioxidants After Short-Term Restriction in C57BL/6 Mice.
Green CL, Mitchell SE, Derous D, Wang Y, Chen L, Han JJ, Promislow DEL, Lusseau D, Douglas A, Speakman JR. Green CL, et al. J Gerontol A Biol Sci Med Sci. 2020 Jan 20;75(2):218-229. doi: 10.1093/gerona/glz023. J Gerontol A Biol Sci Med Sci. 2020. PMID: 31220223 Free PMC article. - Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women.
Redman LM, Huffman KM, Landerman LR, Pieper CF, Bain JR, Muehlbauer MJ, Stevens RD, Wenner BR, Kraus VB, Newgard CB, Kraus WE, Ravussin E. Redman LM, et al. J Clin Endocrinol Metab. 2011 Feb;96(2):E312-21. doi: 10.1210/jc.2010-1971. Epub 2010 Dec 1. J Clin Endocrinol Metab. 2011. PMID: 21123443 Free PMC article. Clinical Trial. - Strain-specific metabolic responses to long-term caloric restriction in female ILSXISS recombinant inbred mice.
Mulvey L, Wilkie SE, Borland G, Griffiths K, Sinclair A, McGuinness D, Watson DG, Selman C. Mulvey L, et al. Mol Cell Endocrinol. 2021 Sep 15;535:111376. doi: 10.1016/j.mce.2021.111376. Epub 2021 Jul 9. Mol Cell Endocrinol. 2021. PMID: 34246728 Free PMC article. Review. - Phytochemicals in regulating fatty acid β-oxidation: Potential underlying mechanisms and their involvement in obesity and weight loss.
Rupasinghe HP, Sekhon-Loodu S, Mantso T, Panayiotidis MI. Rupasinghe HP, et al. Pharmacol Ther. 2016 Sep;165:153-63. doi: 10.1016/j.pharmthera.2016.06.005. Epub 2016 Jun 8. Pharmacol Ther. 2016. PMID: 27288729 Review.
Cited by
- Integrative Hedonic and Homeostatic Food Intake Regulation by the Central Nervous System: Insights from Neuroimaging.
Campos A, Port JD, Acosta A. Campos A, et al. Brain Sci. 2022 Mar 24;12(4):431. doi: 10.3390/brainsci12040431. Brain Sci. 2022. PMID: 35447963 Free PMC article. Review. - The Effects of Pregnancy on Amino Acid Levels and Nitrogen Disposition.
Enthoven LF, Shi Y, Fay EE, Moreni S, Mao J, Honeyman EM, Smith CK, Whittington D, Brockerhoff SE, Isoherranen N, Totah RA, Hebert MF. Enthoven LF, et al. Metabolites. 2023 Feb 7;13(2):242. doi: 10.3390/metabo13020242. Metabolites. 2023. PMID: 36837861 Free PMC article. - Aspirin Metabolites and Mammographic Breast Density in Premenopausal Women.
Singh RK, Getz KR, Kyeyune JK, Jeon MS, Luo C, Luo J, Toriola AT. Singh RK, et al. Cancer Epidemiol Biomarkers Prev. 2024 Aug 1;33(8):1126-1128. doi: 10.1158/1055-9965.EPI-24-0017. Cancer Epidemiol Biomarkers Prev. 2024. PMID: 38700429 - A Metabolomic Analysis of the Sex-Dependent Hispanic Paradox.
Patterson J, Shi X, Bresette W, Eghlimi R, Atlas S, Farr K, Vega-López S, Gu H. Patterson J, et al. Metabolites. 2021 Aug 20;11(8):552. doi: 10.3390/metabo11080552. Metabolites. 2021. PMID: 34436492 Free PMC article. - Genetic and metabolomic architecture of variation in diet restriction-mediated lifespan extension in Drosophila.
Jin K, Wilson KA, Beck JN, Nelson CS, Brownridge GW 3rd, Harrison BR, Djukovic D, Raftery D, Brem RB, Yu S, Drton M, Shojaie A, Kapahi P, Promislow D. Jin K, et al. PLoS Genet. 2020 Jul 9;16(7):e1008835. doi: 10.1371/journal.pgen.1008835. eCollection 2020 Jul. PLoS Genet. 2020. PMID: 32644988 Free PMC article.
References
- Friedman JM. Obesity in the new millennium. Nature. 2000;404(6778):632–634. - PubMed
- Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, Ohannesian JP, Marco CC, McKee LJ, Bauer TL, Caro JF. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334(5):292–295. - PubMed
- Maffei M, Halaas J, Ravussin E, Pratley RE, Lee GH, Zhang Y, Fei H, Kim S, Lallone R, Ranganathan S, Kern PA, Friedman JM. Leptin levels in human and rodent: measurement of plasma leptin and ob RNA in obese and weight-reduced subjects. Nat Med. 1995;1(11):1155–1161. - PubMed
- Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87(5):2391–2394. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources