mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass - PubMed (original) (raw)
Review
mTOR as a Key Regulator in Maintaining Skeletal Muscle Mass
Mee-Sup Yoon. Front Physiol. 2017.
Abstract
Maintenance of skeletal muscle mass is regulated by the balance between anabolic and catabolic processes. Mammalian target of rapamycin (mTOR) is an evolutionarily conserved serine/threonine kinase, and is known to play vital roles in protein synthesis. Recent findings have continued to refine our understanding of the function of mTOR in maintaining skeletal muscle mass. mTOR controls the anabolic and catabolic signaling of skeletal muscle mass, resulting in the modulation of muscle hypertrophy and muscle wastage. This review will highlight the fundamental role of mTOR in skeletal muscle growth by summarizing the phenotype of skeletal-specific mTOR deficiency. In addition, the evidence that mTOR is a dual regulator of anabolism and catabolism in skeletal muscle mass will be discussed. A full understanding of mTOR signaling in the maintenance of skeletal muscle mass could help to develop mTOR-targeted therapeutics to prevent muscle wasting.
Keywords: atrophy; hypertrophy; mTOR; sarcopenia; skeletal muscle.
Figures
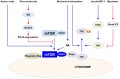
Figure 1
The summary of the regulation of mTORC1 activity in skeletal muscles. Multiple factors and pathways affect mTORC1 activity to regulate skeletal muscle mass. mTORC1 is activated by IGF-I/insulin, mechanical stimulation and amino acids (blue lines) and inhibited by glucocorticoids and myostatin (red lines). Activated mTORC1 increases protein synthesis in skeletal muscle.
Similar articles
- The role of mTORC1 in the regulation of skeletal muscle mass.
Bodine SC. Bodine SC. Fac Rev. 2022 Nov 11;11:32. doi: 10.12703/r/11-32. eCollection 2022. Fac Rev. 2022. PMID: 36532707 Free PMC article. Review. - The mechanistic and ergogenic effects of phosphatidic acid in skeletal muscle.
Shad BJ, Smeuninx B, Atherton PJ, Breen L. Shad BJ, et al. Appl Physiol Nutr Metab. 2015 Dec;40(12):1233-41. doi: 10.1139/apnm-2015-0350. Epub 2015 Sep 21. Appl Physiol Nutr Metab. 2015. PMID: 26566242 Review. - Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo.
Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Bodine SC, et al. Nat Cell Biol. 2001 Nov;3(11):1014-9. doi: 10.1038/ncb1101-1014. Nat Cell Biol. 2001. PMID: 11715023 - Anabolic and catabolic pathways regulating skeletal muscle mass.
McCarthy JJ, Esser KA. McCarthy JJ, et al. Curr Opin Clin Nutr Metab Care. 2010 May;13(3):230-5. doi: 10.1097/MCO.0b013e32833781b5. Curr Opin Clin Nutr Metab Care. 2010. PMID: 20154608 Free PMC article. Review. - Akt signalling through GSK-3beta, mTOR and Foxo1 is involved in human skeletal muscle hypertrophy and atrophy.
Léger B, Cartoni R, Praz M, Lamon S, Dériaz O, Crettenand A, Gobelet C, Rohmer P, Konzelmann M, Luthi F, Russell AP. Léger B, et al. J Physiol. 2006 Nov 1;576(Pt 3):923-33. doi: 10.1113/jphysiol.2006.116715. Epub 2006 Aug 17. J Physiol. 2006. PMID: 16916907 Free PMC article.
Cited by
- Diet-induced impairment of skeletal muscle and adipose tissue metabolic homeostasis and its prevention by probiotic administration.
Di Porzio A, Barrella V, Cigliano L, Mauriello G, Troise AD, Scaloni A, Iossa S, Mazzoli A. Di Porzio A, et al. Pflugers Arch. 2024 Nov 14. doi: 10.1007/s00424-024-03041-9. Online ahead of print. Pflugers Arch. 2024. PMID: 39537965 - Ursolic Acid Restores Redox Homeostasis and Pro-inflammatory Cytokine Production in Denervation-Induced Skeletal Muscle Atrophy.
Yadav A, Dabur R. Yadav A, et al. Appl Biochem Biotechnol. 2024 Oct 3. doi: 10.1007/s12010-024-05059-2. Online ahead of print. Appl Biochem Biotechnol. 2024. PMID: 39361198 - A single-center, double-blind, randomized, placebo-controlled, two-arm study to evaluate the safety and efficacy of once-weekly sirolimus (rapamycin) on muscle strength and endurance in older adults following a 13-week exercise program.
Stanfield B, Kaeberlein M, Leroux B, Jones J, Lucas R, Arroll B. Stanfield B, et al. Trials. 2024 Oct 1;25(1):642. doi: 10.1186/s13063-024-08490-2. Trials. 2024. PMID: 39354527 Free PMC article. - Novel metabolic and lipidomic biomarkers of sarcopenia.
Hsu WH, Wang SY, Chao YM, Chang KV, Han DS, Lin YL. Hsu WH, et al. J Cachexia Sarcopenia Muscle. 2024 Oct;15(5):2175-2186. doi: 10.1002/jcsm.13567. Epub 2024 Aug 21. J Cachexia Sarcopenia Muscle. 2024. PMID: 39169398 Free PMC article. - The immunology of sickness metabolism.
Wensveen FM, Šestan M, Polić B. Wensveen FM, et al. Cell Mol Immunol. 2024 Sep;21(9):1051-1065. doi: 10.1038/s41423-024-01192-4. Epub 2024 Aug 6. Cell Mol Immunol. 2024. PMID: 39107476 Free PMC article. Review.
References
- Allen D. L., Cleary A. S., Hanson A. M., Lindsay S. F., Reed J. M. (2010). CCAAT/enhancer binding protein-δ expression is increased in fast skeletal muscle by food deprivation and regulates myostatin transcription in vitro. Am. J. Physiol. Regul. Integr. Compar. Physiol. 299, R1592–R1601. 10.1152/ajpregu.00247.2010 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous