Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics - PubMed (original) (raw)
Mutual dependence of the MRTF-SRF and YAP-TEAD pathways in cancer-associated fibroblasts is indirect and mediated by cytoskeletal dynamics
Charles T Foster et al. Genes Dev. 2017.
Abstract
Both the MRTF-SRF and the YAP-TEAD transcriptional regulatory networks respond to extracellular signals and mechanical stimuli. We show that the MRTF-SRF pathway is activated in cancer-associated fibroblasts (CAFs). The MRTFs are required in addition to the YAP pathway for CAF contractile and proinvasive properties. We compared MRTF-SRF and YAP-TEAD target gene sets and identified genes directly regulated by one pathway, the other, or both. Nevertheless, the two pathways exhibit mutual dependence. In CAFs, expression of direct MRTF-SRF genomic targets is also dependent on YAP-TEAD activity, and, conversely, YAP-TEAD target gene expression is also dependent on MRTF-SRF signaling. In normal fibroblasts, expression of activated MRTF derivatives activates YAP, while activated YAP derivatives activate MRTF. Cross-talk between the pathways requires recruitment of MRTF and YAP to DNA via their respective DNA-binding partners (SRF and TEAD) and is therefore indirect, arising as a consequence of activation of their target genes. In both CAFs and normal fibroblasts, we found that YAP-TEAD activity is sensitive to MRTF-SRF-induced contractility, while MRTF-SRF signaling responds to YAP-TEAD-dependent TGFβ signaling. Thus, the MRF-SRF and YAP-TEAD pathways interact indirectly through their ability to control cytoskeletal dynamics.
Keywords: MRTF; SRF; TEAD; YAP; cancer-associated fibroblast; mechanotransduction; transcription.
© 2018 Foster et al.; Published by Cold Spring Harbor Laboratory Press.
Figures
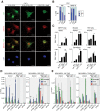
Figure 1.
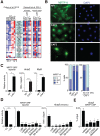
The MRTF–SRF pathway is activated in CAFs. (A) Heat map expression profiles of MRTF–SRF target genes (Esnault et al. 2014) that are overexpressed in CAFs (Calvo et al. 2013). (Left) Fibroblasts from various stages of the mouse PyMT mammary tumor model (Calvo et al. 2013). (Right) NIH3T3 fibroblasts stimulated either with serum; with or without latrunculin B (LatB), which inhibits MRTF activation; or with cytochalasin D (CD), which specifically activates the MRTFs by competing for G-actin binding (Vartiainen et al. 2007). Genes shaded in blue are cytoskeletal components or regulators. (B, top) Immunofluorescence microscopy of MRTF-A in normal mammary fibroblasts (NF1), CAF1, CAF2. (Bottom) MRTF-A localization in NFs and CAFs and in CAF1 cells plated on stiff and soft polyacrylamide hydrogels. (C) Higher cytoplasmic concentration; (N/C) equal concentration over the whole cell; (N) higher nuclear concentration. (C) Comparison of MRTF–SRF reporter gene activity and Acta2 and Myl9 transcripts in NF1 and CAF1 cells. Data are mean ± SEM. n = 3. (*) P < 0.05. (D) CAF1 cells were transfected with MRTF–SRF reporter and C3 transferase expression plasmids and treated with inhibitors as indicated before analysis of reporter activity or Acta2 transcripts. Data are mean ± SEM. n = 3. (***) P < 0.001; (**) P < 0.01; (*) P < 0.05 by _t_-test. (E) ChIP analysis of MRTF-A in inhibitor-treated CAF1 cells. Data are mean ± SEM. (**) P < 0.01; (*) P < 0.05.
Figure 2.
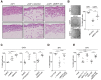
MRTFs are required for CAF matrix remodeling activity and contractility. (A) Representative images of invasion of 4T1 breast carcinoma cells into collagen–Matrigel containing NF1 or CAF1 cells treated with control or MRTF-A/B siRNA as indicated. (B,C) MRTF activity is required for contractility of CAF1 cells plated in collagen–Matrigel. (B) Cells were treated for 72 h with MRTF-A/B siRNA. n = 3, each plated into three gels. (C) Cells were pretreated for 20 h with inhibitors as indicated before plating. n = 3, each plated into two gels. (D,E) Normal fibroblasts exhibit MRTF- and YAP-stimulated contractility. (D) NF1 cells with siMRTF-A/B pretreatment as indicated were stimulated with 15% FCS. n = 4, each plated into four gels. (E) Cells transfected with constitutively active MRTF123-1A or 5SA-YAP expression plasmids with siMRTF-A/B or siYAP as indicated. n = 3, each plated into four gels. Data are mean contraction at 24 h ± SEM. (****) P < 0.0001; (***) P < 0.001, _t_-test.
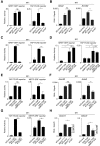
Figure 3.
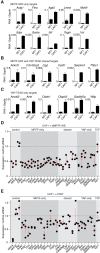
MRTF and YAP direct target genes are activated in CAFs. (A_–_C) Quantitative PCR (qPCR) analysis of MRTF and YAP target gene expression in NF1 and CAF1. Data are mRNA normalized to Gapdh transcripts. Mean ± SEM. n = 3. (A) MRTF-only targets. (B) Shared MRTF/SRF and YAP/TEAD targets. (C) YAP-only targets. (D,E) Mutual dependence of MRTF and YAP target gene expression. RNA from CAF1 cells was treated with siRNA against MRTF-A/B (D) or YAP (E). Data points represent independent siRNA treatments normalized to the geometric mean from triplicate control siRNA treatments. Red lines indicate mean and SEM.
Figure 4.
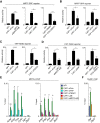
MRTF–SRF reporter gene activity is indirectly dependent on YAP–TEAD function and vice versa. NF1 or CAF1 cells were depleted of MRTFs, SRFs, YAP, or TEAD and transfected with the MRTF–SRF or YAP–TEAD reporters with CCG-203971 treatment and serum stimulation as indicated. Data are means ± SEM. n = 4. (****) P < 0.0001; (***) P < 0.001; (**) P < 0.01; (*) P < 0.05, by _t_-test. (A,B) Elevated MRTF–SRF reporter activity in CAFs. (C,D) Elevated YAP–TEAD reporter activity in CAFs. Note that YAP knockdown alone is sufficient to reduce reporter activity to baseline, suggesting that TAZ does not play a significant role in this system, consistent with previous functional analysis (Calvo et al. 2013). (E,F) ChIP analysis of MRTF-A binding (E) and TEAD1 binding (F). Controls were Egr1 (TCF-SRF target, no MRTF-binding) and Zfp37 (no SRF or TEAD binding). Cells were treated with siRNAs or LatB as indicated. (**) P < 0.01; (*) P < 0.05, by _t_-test.
Figure 5.
MRTF and YAP are independently regulated. (A,B) Immunofluorescence analysis of MRTF-A and YAP in cells treated with 2 µM CD, 1 µM LatB, or DMSO vehicle for 30 min. (A) Representative images. Bar, 20 µm. (B) Quantitation of YAP and MRTF-A subcellular localization from 20 fields of view at 20× magnification. (C) High cytoplasmic concentration; (N/C) equal concentration over the whole cell; (N) high nuclear concentration. (C) qPCR analysis of MRTF and YAP target gene intronic RNA in NF1 and CAF1 cells stimulated with CD for 30 min. Data are means ± SEM. n = 3. (Left) MRTF-only target genes. (Middle) Shared targets. (Right) YAP-only targets. (D) ChIP analysis of MRTF–SRF and YAP–TEAD recruitment to target genes in MDA-MB231 cells treated with CD or LatB for 30 min, as indicated. For target gene details, see
Supplemental Figure S4
. The Zfp37 control does not bind either MRTF–SRF or YAP–TEAD. (D) MRTF-A and SRF binding. (E) YAP and TEAD4 binding. Data are means ± SEM. n = 3 independent chromatin preparations. (**) P < 0.01; (*) P < 0.05 Student's _t_-test, relative to untreated. The sensitivity of SRF binding to drug treatments likely reflects cooperative MRTF–SRF recruitment to promoters (Esnault et al. 2014; Gualdrini et al. 2016); the sensitivity TEAD4 binding to drug treatment is consistent with the YAP dependence of TEAD binding observed previously by others (Stein et al. 2015) and may reflect either cooperative recruitment or nuclear export of TEAD4.
Figure 6.
Constitutively active MRTF and YAP derivatives are not mutually dependent. NF1 cells were depleted of MRTFs, SRFs, YAP, or TEAD, as indicated, and transfected with the MRTF–SRF reporter or YAP–TEAD reporters together with expression plasmids encoding MRTF123-1A, SRF-VP16, or 5SA-YAP, as indicated. Data are means ± SEM. n = 3. (****) P < 0.0001; (***) P < 0.001; (**) P < 0.01; (*) P < 0.05, by Student's _t_-test. (A,B) MRTF123-1A activates the MRTF–SRF reporter (A) and MRTF–SRF target genes (B) independently of YAP. (C) MRTF123-1A requires SRF to activate both the MRTF–SRF and YAP–TEAD reporters. (D) SRF-VP16 does not require MRTFs to activate the MRTF–SRF and YAP–TEAD reporters. (E,F) 5SA-YAP activates the YAP–TEAD reporter (E) and YAP–TEAD target genes (F) independently of MRTFs. (G) 5SA-YAP requires TEAD1 to activate both the MRTF–SRF and YAP–TEAD reporters. (H) Indirect YAP and MRTF signaling contributes to activation of the MRTF–SRF and YAP–TEAD shared target gene Ankrd1 by constitutively active MRTF123-1A or 5SA-YAP. Notional contributions of YAP and MRTF activation are indicated by brackets.
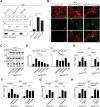
Figure 7.
MRTF activates YAP through cell contractility. (A, left) Constitutively active MRTF123-1A and 5SA-YAP increase the proportion of actin in the pellet (lanes F) compared with soluble fractions (lanes G) in sedimentation assays. 14-3-3 and H3 are controls for soluble and pellet fractions. (Right) Quantitation. Data are means ± SEM. n = 3. (**) P < 0.01, Student's _t_-test. (B) Immunofluorescence analysis of NF1 cells with or without MRTF123-1A expression with 30 min LatB treatment, as indicated. Arrows indicate cells overexpressing MRTF123-1A. Bar, 25 µm. (C) Immunoblot analysis of YAP S127 phosphorylation in NF1 cells expressing MRTF123-1A with 2-h inhibitor treatments, as indicated. (D) Analysis of MRTF–SRF or YAP–TEAD reporter activity in cells expressing MRTF123-1A with inhibitor treatment, as indicated. Data are means ± SEM. n = 3. (**) P < 0.01; (*) P < 0.05. (E_–_G) Cells expressing MRTF123-1A were treated with inhibitors as indicated, and expression of intronic RNA of the MRTF-only target Acta2 and the YAP-only target Amotl2. Data are means ± SEM. n = 3. (***) P < 0.001; (**) P < 0.01; (*) P < 0.05. (H) Analysis of reporter activity in NF1 cells expressing MRTF123-1A with depletion of Cdc42EP3, as indicated. Analysis was as in D.
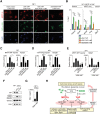
Figure 8.
YAP–TEAD activation of MRTF requires TGF-β signaling. (A) Immunofluorescence analysis of NF1 cells expressing 5SA-YAP or 5SA/S94A-YAP with LatB treatment, as indicated. (B) ChIP analysis of NF1 cells expressing 5SA-YAP or 5SA/S94A-YAP before or after 30 min of LatB treatment. Data are mean ± SEM. (***) P < 0.001; (**) P < 0.01; (*) P < 0.05. (C_–_E) Cells were transfected with the MRTF–SRF reporter and 5SA-YAP or 5SA/S94A-YAP and treated with the indicated inhibitors. Data are mean ± SEM. n = 3. (**) P < 0.01; (*) P < 0.05. (C) Activation of the MRTF reporter and MRTF-only target Acta2 is sensitive to LatB. (D) Activation of the YAP reporter and YAP-only target Amotl2 is not sensitive to LatB. (E) MRTF–SRF reporter activity or Acta2 expression requires F-actin assembly and TGFβ signaling. (F) Immunoblot analysis of YAP, S465/S467-diphosphorylated Smad2, total Smad2, and ERK control in NF1 and CAF1 cells with or without depletion of YAP. (G) Inhba expression levels in NF1 cells with or without 5SA-YAP expression. (H) Indirect cytoskeletal cross-talk model for the mutual dependence of MRTFs and YAP. MRTF–SRF signaling influences YAP activity via mechanisms that include the potentiation of cell contractility; YAP–TEAD signaling influences MRTF activity at least in part through potentiation of TGFβ signaling. Both pathways impinge on septin–actin interaction. Likely target genes involved are shown. For discussion, see the text.
Similar articles
- Mechanosensitive transcriptional coactivators MRTF-A and YAP/TAZ regulate nucleus pulposus cell phenotype through cell shape.
Fearing BV, Jing L, Barcellona MN, Witte SE, Buchowski JM, Zebala LP, Kelly MP, Luhmann S, Gupta MC, Pathak A, Setton LA. Fearing BV, et al. FASEB J. 2019 Dec;33(12):14022-14035. doi: 10.1096/fj.201802725RRR. Epub 2019 Oct 22. FASEB J. 2019. PMID: 31638828 Free PMC article. - TGF-β1 regulates the expression and transcriptional activity of TAZ protein via a Smad3-independent, myocardin-related transcription factor-mediated mechanism.
Miranda MZ, Bialik JF, Speight P, Dan Q, Yeung T, Szászi K, Pedersen SF, Kapus A. Miranda MZ, et al. J Biol Chem. 2017 Sep 8;292(36):14902-14920. doi: 10.1074/jbc.M117.780502. Epub 2017 Jul 24. J Biol Chem. 2017. PMID: 28739802 Free PMC article. - TAZ/YAP fusion proteins: mechanistic insights and therapeutic opportunities.
Garcia K, Gingras AC, Harvey KF, Tanas MR. Garcia K, et al. Trends Cancer. 2022 Dec;8(12):1033-1045. doi: 10.1016/j.trecan.2022.08.002. Epub 2022 Sep 9. Trends Cancer. 2022. PMID: 36096997 Free PMC article. Review. - A combat with the YAP/TAZ-TEAD oncoproteins for cancer therapy.
Pobbati AV, Hong W. Pobbati AV, et al. Theranostics. 2020 Feb 18;10(8):3622-3635. doi: 10.7150/thno.40889. eCollection 2020. Theranostics. 2020. PMID: 32206112 Free PMC article. Review.
Cited by
- Mechanical confinement matters: Unveiling the effect of two-photon polymerized 2.5D and 3D microarchitectures on neuronal YAP expression and neurite outgrowth.
Sharaf A, Frimat JP, Accardo A. Sharaf A, et al. Mater Today Bio. 2024 Nov 2;29:101325. doi: 10.1016/j.mtbio.2024.101325. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39569166 Free PMC article. - STEMIN and YAP5SA, the future of heart repair?
Bejar N, Xiao S, Iyer D, Muili A, Adeleye A, McConnell BK, Schwartz RJ. Bejar N, et al. Exp Biol Med (Maywood). 2024 Oct 31;249:10246. doi: 10.3389/ebm.2024.10246. eCollection 2024. Exp Biol Med (Maywood). 2024. PMID: 39544432 Free PMC article. Review. - mDia2 is an important mediator of MRTF-A-dependent regulation of breast cancer cell migration.
Eder I, Yu V, Antonello J, Chen F, Gau D, Chawla P, Joy M, Lucas PC, Boone D, Lee AV, Roy P. Eder I, et al. Mol Biol Cell. 2024 Oct 1;35(10):ar133. doi: 10.1091/mbc.E24-01-0008. Epub 2024 Aug 28. Mol Biol Cell. 2024. PMID: 39196658 Free PMC article. - Endothelial LATS2 is a suppressor of bone marrow fibrosis.
Sivaraj KK, Majev PG, Dharmalingam B, Schröder S, Banjanin B, Stehling M, Zeuschner D, Nordheim A, Schneider RK, Adams RH. Sivaraj KK, et al. Nat Cardiovasc Res. 2024;3(8):951-969. doi: 10.1038/s44161-024-00508-x. Epub 2024 Jul 29. Nat Cardiovasc Res. 2024. PMID: 39155965 Free PMC article. - Pediatric cancer-pathology and microenvironment influence: a perspective into osteosarcoma and non-osteogenic mesenchymal malignant neoplasms.
Sergi CM. Sergi CM. Discov Oncol. 2024 Aug 18;15(1):358. doi: 10.1007/s12672-024-01240-5. Discov Oncol. 2024. PMID: 39154307 Free PMC article. Review.
References
- Calvo F, Ege N, Grande-Garcia A, Hooper S, Jenkins RP, Chaudhry SI, Harrington K, Williamson P, Moeendarbary E, Charras G, et al. 2013. Mechanotransduction and YAP-dependent matrix remodelling is required for the generation and maintenance of cancer-associated fibroblasts. Nat Cell Biol 15: 637–646. - PMC - PubMed
- Cordenonsi M, Zanconato F, Azzolin L, Forcato M, Rosato A, Frasson C, Inui M, Montagner M, Parenti AR, Poletti A, et al. 2011. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 147: 759–772. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- FC001-190/MRC_/Medical Research Council/United Kingdom
- FC001190/ARC_/Arthritis Research UK/United Kingdom
- FC001-190/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 268690/ERC_/European Research Council/International
- FC001-190/CRUK_/Cancer Research UK/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous