Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration - PubMed (original) (raw)
Biotherapeutic Effect of Gingival Stem Cells Conditioned Medium in Bone Tissue Restoration
Francesca Diomede et al. Int J Mol Sci. 2018.
Abstract
Bone tissue engineering is one of the main branches of regenerative medicine. In this field, the use of a scaffold, which supported bone development, in combination with mesenchymal stem cells (MSCs), has promised better outcomes for bone regeneration. In particular, human gingival mesenchymal stem cells (hGMSCs) may present advantages compared to other MSCs, including the easier isolation. However, MSCs' secretome has attracted much attention for its potential use in tissue regeneration, such as conditioned medium (CM) that contains different soluble factors proved to be useful for the regenerative purposes. In this study, we evaluated the osteogenic capacity of a poly-(lactide) (3D-PLA) scaffold enriched with hGMSCs and hGMSCs derived CM and its ability to regenerate bone defects in rat calvarias. 3D-PLA alone, 3D-PLA + CM or 3D-PLA + hGMSCs with/without CM were implanted in Wistar male rats subjected to calvarial defects. We observed that 3D-PLA scaffold enriched with hGMSCs and CM showed a better osteogenic capacity, being able to repair the calvarial defect as revealed in vivo by morphological evaluation. Moreover, transcriptomic analysis in vitro revealed the upregulation of genes involved in ossification and regulation of ossification in the 3D-PLA + CM + hGMSCs group. All of these results indicate the great osteogenic ability of 3D-PLA + CM + hGMSCs supporting its use in bone regenerative medicine, in particular in the repair of cranial bone defects. Especially, hGMSCs derived CM played a key role in the induction of the osteogenic process and in bone regeneration.
Keywords: biomaterial; conditioned medium; gingival fibroblast; tissue regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Figure 1
Human gingival mesenchymal stem cells (hGMSCs) characterization. (A) flow cytometry showed the positivity expression of Oct3/4, Sox-2, SSEA-4, CD29, CD44, CD73, CD90 and CD105. While haematopoietic markers are not detectable; (B) light inverted microscopy image of plastic-adherent hGMSCs stained with toluidine blue solution; (C) hGMSCs under osteogenic differentiation conditions stained with alizarin red S solution; (D) hGMSCs stained with Adipo Oil red to demonstrate adipogenic differentiation. Scale bars represent 10 μm.
Figure 2
Scanning electron microscope (SEM) detection. (A) three-dimensional scaffold design observed at low magnification (50×). The scale bar represents 100 μm; (B) at high magnification (1000×), the cellular bridge is clearly visible. The scale bar represents 10 μm.
Figure 3
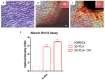
In vitro osteogenic performance. hGMSCs maintained under standard conditions for six weeks and subsequently stained with Alizarin Red S solution. (A) hGMSCs used as control culture; (B) hGMSCs cultured with poly-(lactide) (3D-PLA) scaffold; (C) hGMSCs cultured with 3D-PLA + conditioned medium (CM); (D) histograms of alizarin red S staining quantification. Scale bars represent 10 μm. Graph optical density: ** p < 0.01 was considered statistically significant.
Figure 4
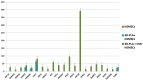
Expression levels of genes differentially expressed in hGMSCs, 3D-PLA + hGMSCs and 3D-PLA + conditioned medium (CM) + hGMSCs (false discovery rate (FDR) < 0.05; n = 3).
Figure 5
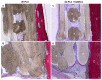
Histological examination of 3D-PLA group and 3D-PLA + hGMSCs group. Representative methylene blue and acid fuchsin images. (A) 3D-PLA group at low magnification (4×); (B) 3D-PLA group at high magnification (20×); (C) 3D-PLA + hGMSCs group at low magnification (4×); (D) 3D-PLA + hGMSCs group at high magnification (20×). Scale bars represent 10 μm. *: 3D-PLA scaffold; C: rat calvaria.
Figure 6
Histological examination of 3D-PLA + CM group and 3D-PLA + CM + hGMSCs group. Representative methylene blue and acid fuchsin images. (A) 3D-PLA + CM group at low magnification (4×); (B) 3D-PLA + CM group at high magnification (20×); (C) 3D-PLA + CM + hGMSCs group at low magnification (4×); (D) 3D-PLA + CM + hGMSCs group at high magnification (20×). *: 3D-PLA scaffold; C: rat calvaria. Scale bars represent 10 μm.
Similar articles
- Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair.
Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A, Nanci A, Caputi S, Fontana A, Mazzon E, Trubiani O. Diomede F, et al. Stem Cell Res Ther. 2018 Apr 13;9(1):104. doi: 10.1186/s13287-018-0850-0. Stem Cell Res Ther. 2018. PMID: 29653587 Free PMC article. - 3D Printing PLA/Gingival Stem Cells/ EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment.
Pizzicannella J, Diomede F, Gugliandolo A, Chiricosta L, Bramanti P, Merciaro I, Orsini T, Mazzon E, Trubiani O. Pizzicannella J, et al. Int J Mol Sci. 2019 Jul 2;20(13):3256. doi: 10.3390/ijms20133256. Int J Mol Sci. 2019. PMID: 31269731 Free PMC article. - Novel cell-free regeneration of bone using stem cell-derived growth factors.
Katagiri W, Osugi M, Kawai T, Ueda M. Katagiri W, et al. Int J Oral Maxillofac Implants. 2013 Jul-Aug;28(4):1009-16. doi: 10.11607/jomi.3036. Int J Oral Maxillofac Implants. 2013. PMID: 23869359 - Oral Bone Tissue Regeneration: Mesenchymal Stem Cells, Secretome, and Biomaterials.
Gugliandolo A, Fonticoli L, Trubiani O, Rajan TS, Marconi GD, Bramanti P, Mazzon E, Pizzicannella J, Diomede F. Gugliandolo A, et al. Int J Mol Sci. 2021 May 15;22(10):5236. doi: 10.3390/ijms22105236. Int J Mol Sci. 2021. PMID: 34063438 Free PMC article. Review. - Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence?
Oryan A, Kamali A, Moshiri A, Baghaban Eslaminejad M. Oryan A, et al. Cells Tissues Organs. 2017;204(2):59-83. doi: 10.1159/000469704. Epub 2017 Jun 24. Cells Tissues Organs. 2017. PMID: 28647733 Review.
Cited by
- Gingival mesenchymal stem cells: Biological properties and therapeutic applications.
Peng Y, Jaar J, Tran SD. Peng Y, et al. J Oral Biol Craniofac Res. 2024 Sep-Oct;14(5):547-569. doi: 10.1016/j.jobcr.2024.07.003. Epub 2024 Jul 13. J Oral Biol Craniofac Res. 2024. PMID: 39108352 Free PMC article. - Functionalizing Collagen Membranes with MSC-Conditioned Media Promotes Guided Bone Regeneration in Rat Calvarial Defects.
Shanbhag S, Kampleitner C, Al-Sharabi N, Mohamed-Ahmed S, Apaza Alccayhuaman KA, Heimel P, Tangl S, Beinlich A, Rana N, Sanz M, Kristoffersen EK, Mustafa K, Gruber R. Shanbhag S, et al. Cells. 2023 Feb 28;12(5):767. doi: 10.3390/cells12050767. Cells. 2023. PMID: 36899904 Free PMC article. - Effect on Osteogenic Differentiation of Genetically Modified IL4 or PDGF-BB Over-Expressing and IL4-PDGF-BB Co-Over-Expressing Bone Marrow-Derived Mesenchymal Stromal Cells In Vitro.
Tsubosaka M, Maruyama M, Huang EE, Zhang N, Utsunomiya T, Gao Q, Shen H, Li X, Kushioka J, Hirata H, Yao Z, Yang YP, Goodman SB. Tsubosaka M, et al. Bioengineering (Basel). 2021 Oct 29;8(11):165. doi: 10.3390/bioengineering8110165. Bioengineering (Basel). 2021. PMID: 34821731 Free PMC article. - Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury.
Mammana S, Gugliandolo A, Cavalli E, Diomede F, Iori R, Zappacosta R, Bramanti P, Conti P, Fontana A, Pizzicannella J, Mazzon E. Mammana S, et al. J Tissue Eng Regen Med. 2019 Jul;13(7):1109-1121. doi: 10.1002/term.2857. Epub 2019 May 31. J Tissue Eng Regen Med. 2019. PMID: 30942960 Free PMC article. - Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration.
Pizzicannella J, Gugliandolo A, Orsini T, Fontana A, Ventrella A, Mazzon E, Bramanti P, Diomede F, Trubiani O. Pizzicannella J, et al. Front Physiol. 2019 Apr 30;10:512. doi: 10.3389/fphys.2019.00512. eCollection 2019. Front Physiol. 2019. PMID: 31114512 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical