Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: importance of KDM6B and SIRT1 for ERβ expression and functionality - PubMed (original) (raw)
Targeting estrogen receptor beta (ERβ) for treatment of ovarian cancer: importance of KDM6B and SIRT1 for ERβ expression and functionality
Giulia Pinton et al. Oncogenesis. 2018.
Abstract
Estrogen receptor (ER) β has growth inhibitory and chemo drug potentiating effect on ovarian cancer cells. We studied the dependence of ERβ function on the presence of KDM6B and SIRT1 in human ovarian cancer cells in vitro. Activation of ERβ with the subtype-selective agonist KB9520 resulted in significant inhibition of human ovarian cancer cell growth. KB9520-activated ERβ had an additive effect on growth inhibition in combination with cisplatin and paclitaxel, respectively. Loss of KDM6B expression had a negative effect on ERβ function as a ligand-dependent inhibitor of ovarian cancer cell growth. In contrast, loss or inhibition of SIRT1 deacetylase activity restored ligand-activated ERβ functionality. Presented data suggest that selective targeting of ERβ with an agonist potentiate chemotherapy efficacy for the treatment of ovarian cancer and that downregulation or inhibition of SIRT1 may further enhance its therapeutic effect.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
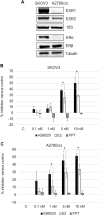
Fig. 1. Estrogen receptor subtype expression and response to ligands.
a Representative RT-PCR and Western blot analyses of ESR1 and ESR2 expression in SKOV3 and A2780cis cells. 18S rRNA and Tubulin were used as controls. b Percentage of growth inhibition in SKOV3 and c A2780cis cells after 24-h treatment with different doses of KB9520, E2, or PPT, in the range of 0.1 to 10 nM. Results are expressed as mean ± s.d. of three independent experiments. *p ≤ 0.05
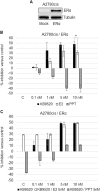
Fig. 2. Response to ligands of ERα-transfected A2780cis cells.
a Representative Western blot analysis of ERα expression in Mock and pcDNA3.1-ERα-transfected A2780cis cells. Tubulin was used as loading controls. b Percentage of growth inhibition in ERα-transfected A2780cis cells after 24-h treatment with different doses of KB9520, E2, or PPT, in the range of 0.1 to 10 nM. c Percentage of growth inhibition in ERα-transfected A2780cis cells after 24-h treatment with different doses of KB9520, in the range of 0.1 to 10 nM, in combination with 5 nM E2 or PPT. Results are expressed as mean ± s.d. of three independent experiments. *p ≤ 0.05
Fig. 3. Agonist-activated ERβ destabilizes the ERα protein.
a Representative Western blot analyses of ERα and ERβ expression in ERα-transfected A2780cis cells after 24-h treatment with 10 nM of KB9520, E2, or PPT. b Representative Western blot analyses of ERα and ERβ expression in ERα-transfected A2780cis cells after 24-h treatment with 10 nM of KB9520 alone or in combination with 5 nM MG132. Tubulin was used as loading control
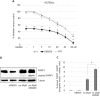
Fig. 4. Additive effect of KB9520 on cisplatin sensitivity in A2780cis cells.
a Percentage of growth inhibition in A2780cis cells after 24-h treatment with different doses of cisplatin, in the range of 1 to 80 μM alone or in combination with 10 nM of KB9520 or PPT. Results are expressed as mean ± s.d. of three independent experiments. *p ≤ 0.05. b Representative Western blot and c densitometric analyses of PARP1 cleavage in A2780cis cells after 24-h treatment with 40 μM of cisplatin alone or in combination with 10 nM of KB9520. *p ≤ 0.05. Tubulin was used as loading control
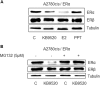
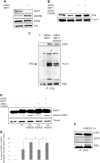
Fig. 5. Role of KDM6B for the expression and function of ERβ and its response to KB9520 in A2780cis cells.
a Representative RT-PCR and b Western blot analyses of KDM6B, SIRT1, and ESR2 expression in A2780cis cells transfected 48 h with non-specific or _KDM6B_-specific siRNAs. 18S rRNA and Tubulin were used as controls. c Immunoprecipitation of ERβ, from lysates of A2780cis cells treated or not with 10 nM of KB9520, 2 h; lysine acetylation and co-immunoprecipitated proteins were detected by Western blot analyses using the respective antibodies (AcLys, ERβ, and p300). d Immunoprecipitation of ERβ, from lysates of A2780cis cells transfected 48 h with non-specific or _KDM6B_-specific siRNAs and treated or not with 10 nM of KB9520, 2 h; lysine acetylation and co-immunoprecipitated proteins were detected by Western blot analyses using the respective antibodies (AcLys, ERβ, and p300). e Representative Western blot analysis of PARP1 cleavage in A2780cis cells transfected with non-specific or _KDM6B-_specific siRNAs after 24 h treatment with 40 μM of cisplatin alone or in combination with 10 nM of KB9520. Tubulin was used as loading control. f Percentage of viable A2780cis cells transfected with non-specific or _KDM6B_-specific siRNAs after 24 h treatment with 40 μM of cisplatin alone or in combination with 10 nM of KB9520. Results are expressed as mean ± s.d. of three independent experiments. *p ≤ 0.05. g Representative RT-PCR, and h respective densitometry, of SIRT1 expression in A2780cis cells transfected with non-specific or _KDM6B_-specific siRNAs for 48 h and then treated or not with 10 nM of KB9520 for 2 h. 18S rRNA was used as housekeeping gene
Fig. 6. Role of SIRT1 for the expression and function of ERβ and its response to KB9520 in A2780cis cells.
a Representative RT-PCR of SIRT1, KDM6B, and ESR2 expression and b Western blot analyses of ERβ expression in A2780cis cells transfected with non-specific or _SIRT1 and KDM6B_-specific siRNAs. 18S rRNA and Tubulin were used as controls. c Immunoprecipitation of ERβ, from lysates of A2780cis cells transfected for 48 h with non-specific or _SIRT1_-specific siRNAs and then treated or not with 10 nM of KB9520, 2 h; lysine acetylation and co-immunoprecipitated proteins were detected by Western blot analyses using the respective antibodies (AcLys, ERβ, and p300). d Representative Western blot and e densitometric analysis of PARP1 cleavage in A2780cis cells transfected with non-specific or SIRT1 and _KDM6B_-specific siRNAs after 24 h treatment with 40 μM of cisplatin alone or in combination with 10 nM of KB9520. Tubulin was used as loading control. *p ≤ 0.05. f Immunoprecipitation of p300, from lysates of A2780cis treated or not 1 h with 10 nM of KB9520; co-immunoprecipitated proteins were detected by Western blot analyses using the respective antibodies (p300, SIRT1, and ERβ)
Similar articles
- Targeting estrogen receptor subtypes (ERα and ERβ) with selective ER modulators in ovarian cancer.
Chan KK, Leung TH, Chan DW, Wei N, Lau GT, Liu SS, Siu MK, Ngan HY. Chan KK, et al. J Endocrinol. 2014 May 12;221(2):325-36. doi: 10.1530/JOE-13-0500. Print 2014 May. J Endocrinol. 2014. PMID: 24819599 - Involvement of estrogen receptor beta in ovarian carcinogenesis.
Bardin A, Hoffmann P, Boulle N, Katsaros D, Vignon F, Pujol P, Lazennec G. Bardin A, et al. Cancer Res. 2004 Aug 15;64(16):5861-9. doi: 10.1158/0008-5472.CAN-04-0552. Cancer Res. 2004. PMID: 15313930 Retracted. - Endogenous estrogen receptor beta is transcriptionally active in primary ovarian cells from estrogen receptor knockout mice.
Mueller SO, Katzenellenbogen JA, Korach KS. Mueller SO, et al. Steroids. 2004 Sep;69(10):681-6. doi: 10.1016/j.steroids.2004.06.004. Steroids. 2004. PMID: 15465114 - Molecular mechanisms of estrogen action: selective ligands and receptor pharmacology.
Katzenellenbogen BS, Choi I, Delage-Mourroux R, Ediger TR, Martini PG, Montano M, Sun J, Weis K, Katzenellenbogen JA. Katzenellenbogen BS, et al. J Steroid Biochem Mol Biol. 2000 Nov 30;74(5):279-85. doi: 10.1016/s0960-0760(00)00104-7. J Steroid Biochem Mol Biol. 2000. PMID: 11162936 Review. - Estrogen receptor beta in the brain: from form to function.
Weiser MJ, Foradori CD, Handa RJ. Weiser MJ, et al. Brain Res Rev. 2008 Mar;57(2):309-20. doi: 10.1016/j.brainresrev.2007.05.013. Epub 2007 Jun 26. Brain Res Rev. 2008. PMID: 17662459 Free PMC article. Review.
Cited by
- Effects of androgens and estrogens on sirtuin 1 gene expression in human aortic endothelial cells.
Tsuchiya T, Takei A, Tsujikado K, Inukai T. Tsuchiya T, et al. Saudi Med J. 2020 Apr;41(4):361-368. doi: 10.15537/smj.2020.4.25006. Saudi Med J. 2020. PMID: 32291422 Free PMC article. - Gene expression and prognosis of sirtuin family members in ovarian cancer.
Zeng Z, Huang Y, Li Y, Huang S, Wang J, Tang Y, Jiang Y. Zeng Z, et al. Medicine (Baltimore). 2020 Jun 12;99(24):e20685. doi: 10.1097/MD.0000000000020685. Medicine (Baltimore). 2020. PMID: 32541517 Free PMC article. - Estrogen Receptor Beta 1: A Potential Therapeutic Target for Female Triple Negative Breast Cancer.
Dey P, Wang A, Ziegler Y, Kumar S, Yan S, Kim SH, Katzenellenbogen JA, Katzenellenbogen BS. Dey P, et al. Endocrinology. 2022 Oct 23;163(12):bqac172. doi: 10.1210/endocr/bqac172. Endocrinology. 2022. PMID: 36251879 Free PMC article. - Synthesis and Discovery of Estra-1,3,5(10),6,8-pentaene-2,16α-diol.
Wai H, Du K, Anesini J, Kim WS, Eastman A, Micalizio GC. Wai H, et al. Org Lett. 2018 Oct 5;20(19):6220-6224. doi: 10.1021/acs.orglett.8b02689. Epub 2018 Sep 17. Org Lett. 2018. PMID: 30221523 Free PMC article. - Estrogen Signaling and Its Potential as a Target for Therapy in Ovarian Cancer.
Langdon SP, Herrington CS, Hollis RL, Gourley C. Langdon SP, et al. Cancers (Basel). 2020 Jun 22;12(6):1647. doi: 10.3390/cancers12061647. Cancers (Basel). 2020. PMID: 32580290 Free PMC article. Review.
References
- Halon A, et al. Loss of estrogen receptor beta expression correlates with shorter overall survival and lack of clinical response to chemotherapy in ovarian cancer patients. Anticancer Res. 2011;31:711–718. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources