Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation - PubMed (original) (raw)
Carnosol Increases Skeletal Muscle Cell Glucose Uptake via AMPK-Dependent GLUT4 Glucose Transporter Translocation
Filip Vlavcheski et al. Int J Mol Sci. 2018.
Abstract
Skeletal muscle is a major insulin-target tissue and plays an important role in glucose homeostasis. Insulin action in muscle activates the phosphatidylinositol-3 kinase (PI3K)/Akt signaling pathway causing the translocation of intracellularly stored GLUT4 glucose transporters to the plasma membrane and increased glucose uptake. Impaired insulin action in muscle results in insulin resistance and type 2 diabetes mellitus (T2DM). Activation of the energy sensor AMP-activated kinase (AMPK) increases muscle glucose uptake and the use of AMPK activators is viewed as an effective strategy to combat insulin resistance. Rosemary extract (RE) has been shown to stimulate muscle AMPK and glucose uptake, but the exact components responsible for these effects are unknown. In the current study, we investigated the effect of carnosol, a RE polyphenol, in L6 rat muscle cells. Carnosol stimulated glucose uptake in L6 myotubes in a dose- and time-dependent manner, did not affect Akt, increased AMPK phosphorylation and plasma membrane GLUT4 levels. The carnosol-stimulated glucose uptake and GLUT4 translocation was significantly reduced by the AMPK inhibitor compound C (CC). Our study is the first to show an AMPK-dependent increase in muscle glucose uptake by carnosol. Carnosol has potential as a glucose homeostasis regulating agent and deserves further study.
Keywords: AMPK; carnosol; glucose uptake; muscle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
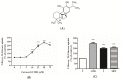
Figure 1
(A) Structure of Carnosol (C20H26O4) (B,C) Effects of carnosol on glucose uptake. (B) Dose response: Serum deprived L6 myotubes were incubated without (0 μM) or with the indicated concentrations of carnosol. (C) Myotubes were incubated without (control, C) or with 25 µM carnosol (COH) (4 h), 100 nM insulin (I) (0.5 h), or 2 mM metformin (MET) (2 h) followed by 2-deoxy-
d
-glucose uptake measurements. Results are the mean ± standard error
(
SE) of three to six independent experiments performed in triplicate. ** p < 0.01, *** p < 0.001, vs. control.
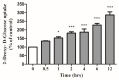
Figure 2
Effects of carnosol on glucose uptake. Time-course: Serum deprived L6 myotubes were incubated without (0) or with 25 µM carnosol for the indicated time followed by 2-deoxy-
d
-glucose uptake measurements. Results are the mean ± SE of three to four independent experiments performed in triplicate. * p < 0.05, *** p < 0.001, vs. control (0 h).
Figure 3
Effect of carnosol on L6 cell morphology. Cells were treated without (Control) or with 25 μM carnosol (COH) for 2 or 12 h. The cells were photographed using EVOS XL Core imaging system at magnification ×20.
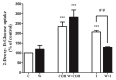
Figure 4
Carnosol-stimulated glucose uptake. Role of PI3K: Myotubes were serum deprived and incubated in the absence (control, C) or presence of 100 nM wortmannin (W) for 15 min followed by treatment with or without 25 µM carnosol (COH) for 4 h, or 100 nM insulin (I) for 0.5 h and 2-deoxy-
d
-glucose uptake measurements. Data are the mean ± SE of three to four experiments performed in triplicate. *** p < 0.001, vs. control (C), ## p < 0.01, vs. insulin (I).
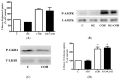
Figure 5
Effects of carnosol on Akt signaling cascade. Whole cell lysates from cells treated without (control, C) or with 25 µM carnosol (COH) (15 min, 2, 6 h), or 100 nM insulin (I) (15 min), were prepared, resolved by SDS-PAGE, and immunoblotted for total Akt, phospho-(Ser473) Akt, total mTOR, phospho-mTOR, or β-actin.
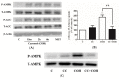
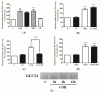
Figure 6
Effects of carnosol on AMP-activated protein kinase (AMPK) signaling cascade. (A) Whole cell lysates from cells treated without (control, C) or with 25 µM carnosol (15 min, 2, 6 h), or 2 mM metformin (MET) (2 h) were prepared, resolved by SDS-PAGE, and immunoblotted for phospho-AMPK, total AMPK, phospho-ACC, total ACC, or β-actin; (B) Cells were incubated in the absence (control, C) or the presence of 25 µM compound (C) (CC) for 0.5 h followed by exposure to 25 µM carnosol (COH) (2.5 h) and 2-deoxy-
d
-glucose uptake measurements. Data are the mean ± SE of three to four experiments performed in triplicate. ** p < 0.01, vs. control (C), ## p < 0.01, vs. carnosol (COH); (C) Whole cell lysates from cells treated without (control, C) or with 25 µM CC for 0.5 h followed by treatment with carnosol (2 h) were prepared, resolved by SDS-PAGE, and immunoblotted for phospho- or total AMPK.
Figure 7
Role of transforming growth factor-β-activated kinase 1 (TAK1), liver kinase B1 (LKB1), and calcium/calmodulin-dependent protein kinase (CaMKK) in the COH-induced effects: (A,D) Myotubes were serum deprived and incubated in the absence (control, C) or presence of 2.5 µM (5_Z_)- oxozeaenol (OZ) (A) or 27 µM STO-609 (STO) (D) for 1 h followed by the addition of 25 µM carnosol (4 h) and 2-deoxy-
d
-glucose uptake measurements. Data are the mean ± SE of two experiments performed in triplicate; (B) Whole cell lysates from cells treated without (control, C) or with 2.5 µM (5_Z_)- oxozeaenol (OZ) 1 h followed by treatment without or with 25 µM carnosol (4 h), were prepared, resolved by SDS-PAGE, and immunoblotted for phospho- or total AMPK (B) or phospho- or total LKB1 (C). ** p < 0.01, vs. control (C).
Figure 8
Effect of carnosol on GLUT4 glucose transporter. GLUT4myc overexpressing L6 myotubes were treated without (control, C) or with 25 µM carnosol) (4 h), 100 nM insulin (I) (0.5 h), 2 mM metformin (MET) (4 h), or 5 µg/mL rosemary extract (RE) (4 h) (A), 100 nM wortmannin (W) for 15 min (B), 25 µM compound C (CC) for 0.5 h (C), or 27 µM STO-609 (STO) for 1 h (D) followed by treatment without or with 25 µM carnosol (4 h). After treatment, GLUT4 transporter translocation measurements were performed. Results are mean ± SE of three to five independent experiments performed in triplicate and expressed as percentage of control, *** p < 0.001, ## p < 0.01. (E) Whole cell lysates from L6 parental myotubes treated without (control, C) or with 25 µM carnosol for 2, 6, and 12 h were prepared, resolved by SDS-PAGE, and immunoblotted for total GLUT4.
Similar articles
- Propolis extract promotes translocation of glucose transporter 4 and glucose uptake through both PI3K- and AMPK-dependent pathways in skeletal muscle.
Ueda M, Hayashibara K, Ashida H. Ueda M, et al. Biofactors. 2013 Jul-Aug;39(4):457-66. doi: 10.1002/biof.1085. Epub 2013 Jan 28. Biofactors. 2013. PMID: 23355380 - Increased skeletal muscle glucose uptake by rosemary extract through AMPK activation.
Naimi M, Tsakiridis T, Stamatatos TC, Alexandropoulos DI, Tsiani E. Naimi M, et al. Appl Physiol Nutr Metab. 2015 Apr;40(4):407-13. doi: 10.1139/apnm-2014-0430. Epub 2014 Dec 22. Appl Physiol Nutr Metab. 2015. PMID: 25794239 - Carnosic acid as a component of rosemary extract stimulates skeletal muscle cell glucose uptake via AMPK activation.
Naimi M, Vlavcheski F, Murphy B, Hudlicky T, Tsiani E. Naimi M, et al. Clin Exp Pharmacol Physiol. 2017 Jan;44(1):94-102. doi: 10.1111/1440-1681.12674. Clin Exp Pharmacol Physiol. 2017. PMID: 27716981 - Trafficking pathway of GLUT4 glucose transporters in muscle (review).
Zorzano A, Sevilla L, Tomàs E, Camps M, Gumà A, Palacín M. Zorzano A, et al. Int J Mol Med. 1998 Sep;2(3):263-71. doi: 10.3892/ijmm.2.3.263. Int J Mol Med. 1998. PMID: 9855697 Review. - Glucose transporter 4: Insulin response mastermind, glycolysis catalyst and treatment direction for cancer progression.
Chang YC, Chan MH, Yang YF, Li CH, Hsiao M. Chang YC, et al. Cancer Lett. 2023 Jun 1;563:216179. doi: 10.1016/j.canlet.2023.216179. Epub 2023 Apr 14. Cancer Lett. 2023. PMID: 37061122 Review.
Cited by
- Carnosic Acid (CA) Induces a Brown Fat-like Phenotype, Increases Mitochondrial Biogenesis, and Activates AMPK in 3T3-L1 Adipocytes.
Vlavcheski F, MacPherson REK, Fajardo V, Sze N, Tsiani E. Vlavcheski F, et al. Biomedicines. 2024 Jul 15;12(7):1569. doi: 10.3390/biomedicines12071569. Biomedicines. 2024. PMID: 39062141 Free PMC article. - New Treatment for Type 2 Diabetes Mellitus Using a Novel Bipyrazole Compound.
Alqudah A, Qnais EY, Wedyan MA, Altaber S, Abudalo R, Gammoh O, Alkhateeb H, Bataineh S, Athamneh RY, Oqal M, Abu-Safieh K, McClements L. Alqudah A, et al. Cells. 2023 Jan 9;12(2):267. doi: 10.3390/cells12020267. Cells. 2023. PMID: 36672202 Free PMC article. - Protein posttranslational modifications in metabolic diseases: basic concepts and targeted therapies.
Yang Y, Wu J, Zhou W, Ji G, Dang Y. Yang Y, et al. MedComm (2020). 2024 Sep 30;5(10):e752. doi: 10.1002/mco2.752. eCollection 2024 Oct. MedComm (2020). 2024. PMID: 39355507 Free PMC article. Review. - New Molecules of Diterpene Origin with Inhibitory Properties toward α-Glucosidase.
Tretyakova E, Smirnova I, Kazakova O, Nguyen HTT, Shevchenko A, Sokolova E, Babkov D, Spasov A. Tretyakova E, et al. Int J Mol Sci. 2022 Nov 4;23(21):13535. doi: 10.3390/ijms232113535. Int J Mol Sci. 2022. PMID: 36362322 Free PMC article. - A systematic analysis of anti-diabetic medicinal plants from cells to clinical trials.
Omale S, Amagon KI, Johnson TO, Bremner SK, Gould GW. Omale S, et al. PeerJ. 2023 Jan 5;11:e14639. doi: 10.7717/peerj.14639. eCollection 2023. PeerJ. 2023. PMID: 36627919 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical