VP1 Amino Acid Residue 145 of Enterovirus 71 Is a Key Residue for Its Receptor Attachment and Resistance to Neutralizing Antibody during Cynomolgus Monkey Infection - PubMed (original) (raw)
Comparative Study
. 2018 Jul 17;92(15):e00682-18.
doi: 10.1128/JVI.00682-18. Print 2018 Aug 1.
Yui Sudaka 1, Ayako Takashino 1, Kyousuke Kobayashi 1, Chikako Kataoka 2, Tadaki Suzuki 3, Naoko Iwata-Yoshikawa 3, Osamu Kotani 4, Yasushi Ami 5, Hiroyuki Shimizu 2, Noriyo Nagata 3, Katsumi Mizuta 6, Yoko Matsuzaki 7, Satoshi Koike 8
Affiliations
- PMID: 29848582
- PMCID: PMC6052295
- DOI: 10.1128/JVI.00682-18
Comparative Study
VP1 Amino Acid Residue 145 of Enterovirus 71 Is a Key Residue for Its Receptor Attachment and Resistance to Neutralizing Antibody during Cynomolgus Monkey Infection
Ken Fujii et al. J Virol. 2018.
Abstract
Enterovirus 71 (EV71) is a causative agent of hand, foot, and mouth disease and sometimes causes severe or fatal neurological complications. The amino acid at VP1-145 determines the virological characteristics of EV71. Viruses with glutamic acid (E) at VP1-145 (VP1-145E) are virulent in neonatal mice and transgenic mice expressing human scavenger receptor B2, whereas those with glutamine (Q) or glycine (G) are not. However, the contribution of this variation to pathogenesis in humans is not fully understood. We compared the virulence of VP1-145E and VP1-145G viruses of Isehara and C7/Osaka backgrounds in cynomolgus monkeys. VP1-145E, but not VP1-145G, viruses induced neurological symptoms. VP1-145E viruses were frequently detected in the tissues of infected monkeys. VP1-145G viruses were detected less frequently and disappeared quickly. Instead, mutants that had a G-to-E mutation at VP1-145 emerged, suggesting that VP1-145E viruses have a replication advantage in the monkeys. This is consistent with our hypothesis proposed in the accompanying paper (K. Kobayashi, Y. Sudaka, A. Takashino, A. Imura, K. Fujii, and S. Koike, J Virol 92:e00681-18, 2018, https://doi.org/10.1128/JVI.00681-18) that the VP1-145G virus is attenuated due to its adsorption by heparan sulfate. Monkeys infected with both viruses produced neutralizing antibodies before the onset of the disease. Interestingly, VP1-145E viruses were more resistant to neutralizing antibodies than VP1-145G viruses in vitro A small amount of neutralizing antibody raised in the early phase of infection may not be sufficient to block the dissemination of VP1-145E viruses. The different resistance of the VP1-145 variants to neutralizing antibodies may be one of the reasons for the difference in virulence.IMPORTANCE The contribution of VP1-145 variants in humans is not fully understood. In some studies, VP1-145G/Q viruses were isolated more frequently from severely affected patients than from mildly affected patients, suggesting that VP1-145G/Q viruses are more virulent. In the accompanying paper (K. Kobayashi, Y. Sudaka, A. Takashino, A. Imura, K. Fujii, and S. Koike, J Virol 92:e00681-18, 2018, https://doi.org/10.1128/JVI.00681-18), we showed that VP1-145E viruses are more virulent than VP1-145G viruses in human SCARB2 transgenic mice. Heparan sulfate acts as a decoy to specifically trap the VP1-145G viruses and leads to abortive infection. Here, we demonstrated that VP1-145G was attenuated in cynomolgus monkeys, suggesting that this hypothesis is also true in a nonhuman primate model. VP1-145E viruses, but not VP1-145G viruses, were highly resistant to neutralizing antibodies. We propose the difference in resistance against neutralizing antibodies as another mechanism of EV71 virulence. In summary, VP1-145 contributes to virulence determination by controlling attachment receptor usage and antibody sensitivity.
Keywords: animal models; enterovirus; neutralizing antibodies; pathogenesis.
Copyright © 2018 American Society for Microbiology.
Figures
FIG 1
Experimental infection of cynomolgus monkeys with VP1-145 mutants and time course of clinical observations. (A) Experimental schedule. Monkeys were intravenously inoculated with 1 ml of virus solution containing 106 TCID50 of VP1-145G or VP1-145E virus at day 0, and the clinical manifestations were observed daily for 10 days. Under anesthesia, clinical samples were collected on indicated days, and postmortem tissue samples were collected at 10 dpi. (B) Monkeys 5212, 5213, 5214, and 5215 were inoculated with Isehara-G and monkeys 5216, 5217, 5218, and 5219 with Isehara-E. (C) Monkeys 5254, 5255, and 5256 were inoculated with C7-G and monkeys 5257, 5258, and 5220 with C7-E.
FIG 2
Histopathological comparison of CNS tissues in EV71-inoculated monkeys. (A) Distribution of EV71-induced lesions. Postmortem CNS tissues at 10 dpi from monkeys inoculated with Isehara or C7/Osaka are shown. Filled triangles and circles indicate viral antigen-positive and neuronal damage, respectively. The gray areas show inflammation in the parenchyma and the meninges of the CNS. (B) Histopathological findings in spinal cord. Typical histopathological changes in the spinal cords of VP1-145E-inoculated groups are shown. Upper panels, histology of the lumbar part of the spinal cord of a monkey inoculated with Isehara-G (monkey 5215) and monkeys inoculated with Isehara-E (5217 and 5218) or C7-E (5257 and 5220), stained using H&E. Lower panels, higher magnifications of the boxes in the anterior horns in the upper panels. Prominent inflammatory infiltrations are indicated by arrowheads. Neuronal degenerations were seen in the anterior horns of the spinal cords within the lesion (arrows).
FIG 3
Schematic illustration of EV71 detection. Virus isolation and viral RNA detection from clinical samples and autopsy samples are summarized. The VP1 region of the viral RNA was amplified, and the sequence was determined. The samples positive for virus isolation and viral RNA with G at VP1-145 are indicated by blue. The samples positive for virus isolation and viral RNA, or viral RNA alone, with E are indicated by red and pink, respectively. The numbers in the boxes indicate the samples containing mutations in the VP1 region, which are summarized in Table 1.
FIG 4
Serum cytokine levels in the inoculated monkeys. (A and B) Production of IFN-α2 in culture supernatant of COS7 cells infected with VP1-145 variants of the Isehara (A) or C7/Osaka (B) strain was analyzed using ELISA. The means ± standard error for the two experiments are shown. (C and D) Serum levels of IFN-α2 collected from the monkeys inoculated with the Isehara (C) or C7/Osaka (D) strain were analyzed using ELISA. (E and F) Serum levels of the cytokines IFN-γ, TNF-α, G-CSF, and IL-6 in the monkeys inoculated with the Isehara (E) or C7/Osaka (F) strain were analyzed using Luminex 200. Serum cytokine levels, collected at 0 (preinoculation) and 1, 4, 7, and 10 dpi, from the VP1-145G virus-inoculated group are indicated in blue, and those from the VP1-145E virus-inoculated group are indicated in red.
FIG 5
HS expression in cynomolgus monkeys. Serial tissue sections of liver (A and B), kidney (C and D), and spinal cord (E and F) prepared from monkeys 5215 and 5254 were stained using H&E (A, C, and E) or subjected to immunofluorescence staining with anti-HS (red) and anti-hSCARB2 (green) (B, D, and F). Nuclei are stained with DAPI (blue). Blood vessels are indicated with asterisks (A to F). Sinusoidal endothelium (arrowheads in panels A and B), renal tubules (arrowheads in panels C and D), glomeruli (arrows in panels C and D), and neuronal cells (arrowheads in panels E and F) are labeled. The area surrounding the renal tubules marked by a dashed box in D is enlarged in the inset. Scale bars indicate 50 μm.
FIG 6
Neutralization titer in sera of monkeys inoculated with the Isehara (A) or C7/Osaka (B) strain of EV71. Serum samples were 4-fold serially diluted (1:4 to 1:256), and PRNT was performed using corresponding VP1-145G and VP1-145E viruses as challenge viruses. The highest serum dilution that achieved more than 80% plaque reduction was defined as the neutralizing titer. The neutralizing titers against VP1-145G and VP1-145E are shown in the left (versus VP1-145G) and right (versus VP1-145E) panels, respectively.
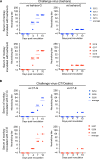
FIG 7
Neutralization sensitivity of VP1-145G and VP1-145E viruses. Dose-response neutralization profiles were generated by incubating VP1-145 variants of Isehara (A), C7/Osaka (B), or SK-EV006 (C) with serial dilutions of α-1095 rabbit antiserum, α-Y90-3205 rabbit antiserum, or MAB979 mouse monoclonal antibody for 2 h at 37°C, followed by plaque assay. The number of plaques was counted, and the resulting data were fit by nonlinear regression to determine the ND80. Error bars represent the standard error from two experiments. Dashed lines indicate the ND80. The means of ND80 ± standard error against VP1-145G and VP1-145E are shown in blue and red, respectively, below each panel. The significance of differences between ND80 against the VP1-145G and VP1-145E viruses was determined using Student's t test, and P values are indicated by asterisks (*, P < 0.5; **, P < 0.05).
Similar articles
- Amino Acid Variation at VP1-145 of Enterovirus 71 Determines Attachment Receptor Usage and Neurovirulence in Human Scavenger Receptor B2 Transgenic Mice.
Kobayashi K, Sudaka Y, Takashino A, Imura A, Fujii K, Koike S. Kobayashi K, et al. J Virol. 2018 Jul 17;92(15):e00681-18. doi: 10.1128/JVI.00681-18. Print 2018 Aug 1. J Virol. 2018. PMID: 29848584 Free PMC article. - The Role of VP1 Amino Acid Residue 145 of Enterovirus 71 in Viral Fitness and Pathogenesis in a Cynomolgus Monkey Model.
Kataoka C, Suzuki T, Kotani O, Iwata-Yoshikawa N, Nagata N, Ami Y, Wakita T, Nishimura Y, Shimizu H. Kataoka C, et al. PLoS Pathog. 2015 Jul 16;11(7):e1005033. doi: 10.1371/journal.ppat.1005033. eCollection 2015 Jul. PLoS Pathog. 2015. PMID: 26181772 Free PMC article. - Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement.
Huang SW, Wang YF, Yu CK, Su IJ, Wang JR. Huang SW, et al. Virology. 2012 Jan 5;422(1):132-43. doi: 10.1016/j.virol.2011.10.015. Epub 2011 Nov 9. Virology. 2012. PMID: 22078110 - Update on enterovirus 71 infection.
Huang PN, Shih SR. Huang PN, et al. Curr Opin Virol. 2014 Apr;5:98-104. doi: 10.1016/j.coviro.2014.03.007. Epub 2014 Apr 12. Curr Opin Virol. 2014. PMID: 24727707 Review. - Cellular receptors for enterovirus A71.
Kobayashi K, Koike S. Kobayashi K, et al. J Biomed Sci. 2020 Jan 10;27(1):23. doi: 10.1186/s12929-020-0615-9. J Biomed Sci. 2020. PMID: 31924205 Free PMC article. Review.
Cited by
- The effects of SCARB2 and SELPLG gene polymorphisms on EV71 infection in hand, foot and mouth disease.
Duan FY, Du ZQ, Wang Y, Luo L, Du LJ, Jiang H, Ma Y, Yang YL. Duan FY, et al. Biomol Biomed. 2023 Sep 4;23(5):815-824. doi: 10.17305/bb.2023.8948. Biomol Biomed. 2023. PMID: 37078358 Free PMC article. - A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism.
Tseligka ED, Sobo K, Stoppini L, Cagno V, Abdul F, Piuz I, Meylan P, Huang S, Constant S, Tapparel C. Tseligka ED, et al. PLoS Pathog. 2018 Aug 3;14(8):e1007190. doi: 10.1371/journal.ppat.1007190. eCollection 2018 Aug. PLoS Pathog. 2018. PMID: 30075025 Free PMC article. - Novel virulence determinants in VP1 regulate the assembly of enterovirus-A71.
Zhang W, Li Q, Yi D, Zheng R, Liu G, Liu Q, Guo S, Zhao J, Wang J, Ma L, Ding J, Zhou R, Ren Y, Sun T, Zhang A, Li X, Zhang Y, Cen S. Zhang W, et al. J Virol. 2024 Dec 17;98(12):e0165524. doi: 10.1128/jvi.01655-24. Epub 2024 Nov 13. J Virol. 2024. PMID: 39535185 Free PMC article. - Molecular characteristics of a coxsackievirus A12 strain in Zhejiang of China, 2019.
Hu L, Zhou L, Wang P, Maimaiti H, Lu Y. Hu L, et al. Virol J. 2022 Oct 12;19(1):160. doi: 10.1186/s12985-022-01892-1. Virol J. 2022. PMID: 36224635 Free PMC article. - Virus adaptation to heparan sulfate comes with capsid stability tradeoff.
Tee HK, Crouzet S, Muliyil A, Mathez G, Cagno V, Dal Peraro M, Antanasijevic A, Clément S, Tapparel C. Tee HK, et al. Elife. 2024 Dec 23;13:e98441. doi: 10.7554/eLife.98441. Elife. 2024. PMID: 39714930 Free PMC article.
References
- Pallansch M, Oberste MS, Whitton JL. 2013. Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses, p 490–530. In Knipe D, Howley PM (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
- Wang X, Peng W, Ren J, Hu Z, Xu J, Lou Z, Li X, Yin W, Shen X, Porta C, Walter TS, Evans G, Axford D, Owen R, Rowlands DJ, Wang J, Stuart DI, Fry EE, Rao Z. 2012. A sensor-adaptor mechanism for enterovirus uncoating from structures of EV71. Nat Struct Mol Biol 19:424–429. doi:10.1038/nsmb.2255. - DOI - PMC - PubMed
- Yamayoshi S, Iizuka S, Yamashita T, Minagawa H, Mizuta K, Okamoto M, Nishimura H, Sanjoh K, Katsushima N, Itagaki T, Nagai Y, Fujii K, Koike S. 2012. Human SCARB2-dependent infection by coxsackievirus A7, A14, and A16 and enterovirus 71. J Virol 86:5686–5696. doi:10.1128/JVI.00020-12. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous