Protein disorder-order interplay to guide the growth of hierarchical mineralized structures - PubMed (original) (raw)
doi: 10.1038/s41467-018-04319-0.
Sherif Elsharkawy 1 2 3, Maria F Pantano 5, Esther Tejeda-Montes 2, Khushbu Mehta 2, Hasan Jamal 2, Shweta Agarwal 6 7 8, Kseniya Shuturminska 1 3, Alistair Rice 6, Nadezda V Tarakina 2, Rory M Wilson 2 4, Andy J Bushby 2 4, Matilde Alonso 9, Jose C Rodriguez-Cabello 9, Ettore Barbieri 2 10, Armando Del Río Hernández 6, Molly M Stevens 6 7 8, Nicola M Pugno 2 5 11, Paul Anderson 1 3, Alvaro Mata 12 13
Affiliations
- PMID: 29858566
- PMCID: PMC5984621
- DOI: 10.1038/s41467-018-04319-0
Protein disorder-order interplay to guide the growth of hierarchical mineralized structures
Sherif Elsharkawy et al. Nat Commun. 2018.
Abstract
A major goal in materials science is to develop bioinspired functional materials based on the precise control of molecular building blocks across length scales. Here we report a protein-mediated mineralization process that takes advantage of disorder-order interplay using elastin-like recombinamers to program organic-inorganic interactions into hierarchically ordered mineralized structures. The materials comprise elongated apatite nanocrystals that are aligned and organized into microscopic prisms, which grow together into spherulite-like structures hundreds of micrometers in diameter that come together to fill macroscopic areas. The structures can be grown over large uneven surfaces and native tissues as acid-resistant membranes or coatings with tuneable hierarchy, stiffness, and hardness. Our study represents a potential strategy for complex materials design that may open opportunities for hard tissue repair and provide insights into the role of molecular disorder in human physiology and pathology.
Conflict of interest statement
The authors declare no competing interests.
Figures
Fig. 1
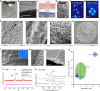
ELR spherulites and hierarchical mineralized structures. a Photograph of a transparent, robust, and flexible statherin-ELR membrane before mineralization. The membrane’s cross-section before mineralization b imaged by SEM and c histologically stained displaying a positive staining for both β-amyloid fibers and d elastin fibers with Congo Red and Elastin von Gieson stains, respectively. e SEM image of the membrane’s cross-section comprising of ELR nanofibers. f, g Polarized light microscope (PLM) images depicting the presence of ELR spherulites with the characteristic birefringent Maltese-cross pattern on the surface and within the bulk of the membranes. h-k SEM images of the top of an RGDS-ELR membrane after mineralization showing the hierarchical organization of the mineralized structures including h aligned fluorapatite nanocrystals that are i, j grouped into prism-like microstructures that further grow into k macroscopic circular structures. l, m The hierarchical structures grow until they meet each other, and n can mineralize completely thin membranes. o Rietveld refinement of an XRD pattern of mineralized membranes showing the fluorapatite nature of the crystalline phase with the typical Bragg peaks of apatite. p 19F solid-sate MAS-NMR spectra confirming the presence of fluorapatite and CaF2 (fluorite) phase at – 103 and – 108 p.p.m., respectively, with increasing fluorapatite peak intensity on the mineralized membrane (green) compared with those without the ELP membrane (red) at the same conditions. q Young’s modulus and hardness relationship between the mineralized structures and different mineralized tissues. Scale bars: a 5 mm; b 20 µm; c, d 40 µm; e 200 nm; f 10 µm; g 3 µm; h 200 nm; i 1 µm; j 10 µm; k 20 µm; l 30 µm; l (inset) 20 µm; m 5 µm; n 20 µm
Fig. 2
Bulk characterization. Organic–inorganic interactions within the bulk of the mineralized membranes. SEM observations revealed a the abundant presence of a dense pattern of spherulite-like structures with b a granulated central region at the bulk of the membranes’ cross-sections, which c template the growth of fluorapatite spherulites. d Near the membrane surface, mineralized structures with nanocrystals grow vertically toward the surface of the membrane. DDC-SEM images of e the core of fluorapatite spherulite structures located closer to the surface of the membrane and f deeper within the bulk of the membrane, revealing a thin low-density material (green) surrounding a denser (orange) one. g Backscattered electron (BSE) image showing brighter areas at the center of the structures, indicating the presence of mineral deep within the membrane. h, i FIB sectioning of the hierarchically mineralized structure resolving the deeper mineralized core structures located underneath the center of the structures. j, kTEM images from a FIB milling lift-out of a mineralized structure illustrating j the change in growth direction of the nanocrystals from parallel to the surface towards the bulk of the ELR membrane, and k the growing interface between the flat-ended inorganic crystals and the organic ELR material. l HRTEM image of a single fluorapatite crystal showing its growth orientation and crystal lattices. m SAED and n FFT analyses showing the characteristic diffraction pattern of the fluorapatite crystals (left) and its 10° co-alignment, respectively. Scale bars: a 2 µm; b, c 1 µm; d 2 µm; e, f 200 nm; g 20 µm; h, i 5 µm; j 200 nm; k 100 nm; l 10 nm; m 3 nm−1
Fig. 3
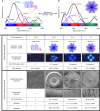
Protein disorder–order optimization and process tuneability. a, b Graphs showing the different levels of ELR order and disorder as a function of cross-linking. The levels of ELR ordered β-sheet structure and disordered random coil can be modulated and tuned, while maintaining β-turn and α-helix conformations nearly constant. c Table summarizing the tuneability of the process including during both ELR matrix assembly (Stage I) and the CaP nucleation and growth (Stage II). Scale bars: 3 µm (ELR spherulite morphology), 20 µm (apatite hierarchical structures), and 200 nm (nanocrystal density)
Fig. 4
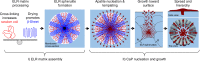
Mechanism of formation. A schematic illustrating the two stages of the proposed mechanism divided in (I) ELR matrix assembly and (II) calcium phosphate (CaP) nucleation and growth
Fig. 5
Resemblance between enamel and the hierarchical structures. SEM images illustrating the close resemblance of human dental enamel a to the hierarchically ordered mineralized structures b grown on a RGDS-ELR membrane at multiple length scales. Scale bars: a, b 200 nm; c, d 1 µm; and e, f 20 µm
Fig. 6
Dental applications of the hierarchical structures. a Application of the in-situ cross-linked ELR membrane conformed over the rough and uneven surface of exposed human dentin, exhibiting the hierarchical mineralized structures as a coating on top of the native tissue. SEM images depicting b a removed section (white square) of the mineralized membrane with c the nanocrystals infiltrating, binding, and occluding the open dentinal tubule structures. d FIB milling of the mineralized coating at different depths to observe the dentin-membrane interface, which exhibits infiltration of nanocrystals occluding the dentinal tubules. e SEM images revealing the effect of the acid attack at different time-points (15 min, and 7 days) on both human dental enamel and the hierarchical mineralized structures. f Graph illustrating the Young’s modulus and hardness of the human enamel and mineralized structures after the acid attack. g DDC-SEM images of the hierarchical mineralized structures after the enzymatic digestion, showing a partially remaining organic material (arrow). Scale bars: a, b 50 µm; c 3 µm; d 10 µm; e 1 µm; g 500 nm. Error bars are represented as SD, n = 10
Similar articles
- Biomimetic Mineralization of Recombinamer-Based Hydrogels toward Controlled Morphologies and High Mineral Density.
Li Y, Chen X, Fok A, Rodriguez-Cabello JC, Aparicio C. Li Y, et al. ACS Appl Mater Interfaces. 2015 Nov 25;7(46):25784-92. doi: 10.1021/acsami.5b07628. Epub 2015 Nov 11. ACS Appl Mater Interfaces. 2015. PMID: 26516652 Free PMC article. - Agarose hydrogel biomimetic mineralization model for the regeneration of enamel prismlike tissue.
Cao Y, Mei ML, Li QL, Lo EC, Chu CH. Cao Y, et al. ACS Appl Mater Interfaces. 2014 Jan 8;6(1):410-20. doi: 10.1021/am4044823. Epub 2013 Dec 26. ACS Appl Mater Interfaces. 2014. PMID: 24354267 - Biomimetic calcium phosphate mineralization with multifunctional elastin-like recombinamers.
Prieto S, Shkilnyy A, Rumplasch C, Ribeiro A, Arias FJ, Rodríguez-Cabello JC, Taubert A. Prieto S, et al. Biomacromolecules. 2011 May 9;12(5):1480-6. doi: 10.1021/bm200287c. Epub 2011 Apr 12. Biomacromolecules. 2011. PMID: 21438535 - The role of extracellular matrix components in dentin mineralization.
Boskey AL. Boskey AL. Crit Rev Oral Biol Med. 1991;2(3):369-87. doi: 10.1177/10454411910020030501. Crit Rev Oral Biol Med. 1991. PMID: 1654141 Review. - The structural biology of the developing dental enamel matrix.
Fincham AG, Moradian-Oldak J, Simmer JP. Fincham AG, et al. J Struct Biol. 1999 Jun 30;126(3):270-99. doi: 10.1006/jsbi.1999.4130. J Struct Biol. 1999. PMID: 10441532 Review.
Cited by
- Phosphorylated and Phosphonated Low-Complexity Protein Segments for Biomimetic Mineralization and Repair of Tooth Enamel.
Chang R, Liu YJ, Zhang YL, Zhang SY, Han BB, Chen F, Chen YX. Chang R, et al. Adv Sci (Weinh). 2022 Feb;9(6):e2103829. doi: 10.1002/advs.202103829. Epub 2022 Jan 2. Adv Sci (Weinh). 2022. PMID: 34978158 Free PMC article. - Application of Thermoresponsive Intrinsically Disordered Protein Polymers in Nanostructured and Microstructured Materials.
Wang B, Patkar SS, Kiick KL. Wang B, et al. Macromol Biosci. 2021 Sep;21(9):e2100129. doi: 10.1002/mabi.202100129. Epub 2021 Jun 18. Macromol Biosci. 2021. PMID: 34145967 Free PMC article. Review. - Peptide-protein coassembling matrices as a biomimetic 3D model of ovarian cancer.
Hedegaard CL, Redondo-Gómez C, Tan BY, Ng KW, Loessner D, Mata A. Hedegaard CL, et al. Sci Adv. 2020 Oct 2;6(40):eabb3298. doi: 10.1126/sciadv.abb3298. Print 2020 Oct. Sci Adv. 2020. PMID: 33008910 Free PMC article. - Skeletal development in the sea urchin relies upon protein families that contain intrinsic disorder, aggregation-prone, and conserved globular interactive domains.
Pendola M, Jain G, Evans JS. Pendola M, et al. PLoS One. 2019 Oct 1;14(10):e0222068. doi: 10.1371/journal.pone.0222068. eCollection 2019. PLoS One. 2019. PMID: 31574084 Free PMC article. - Amelogenin Peptide-Chitosan Hydrogel for Biomimetic Enamel Regrowth.
Mukherjee K, Chakraborty A, Sandhu G, Naim S, Nowotny EB, Moradian-Oldak J. Mukherjee K, et al. Front Dent Med. 2021;2:697544. doi: 10.3389/fdmed.2021.697544. Epub 2021 Jun 16. Front Dent Med. 2021. PMID: 37900722 Free PMC article.
References
- Lakes R. Materials with structural hierarchy. Nature. 1993;361:511–515. doi: 10.1038/361511a0. - DOI
- Weiner S, Addadi L. Design strategies in mineralized biological materials. J. Mater. Chem. 1997;7:689–702. doi: 10.1039/a604512j. - DOI
- Aizenberg J, Fratzl P. Biological and biomimetic materials. Adv. Mater. 2009;21:187–188. doi: 10.1002/adma.200803699. - DOI
- Addadi L, Weiner S. Control and design principles in biological mineralization. Angew. Chem. Int. Ed. Engl. 1992;31:153–169. doi: 10.1002/anie.199201531. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical