Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial - PubMed (original) (raw)
Randomized Controlled Trial
. 2019 Mar;49(4):655-663.
doi: 10.1017/S0033291718001356. Epub 2018 Jun 15.
Dayanna Barreto 2, Heloisa Onias 1, Katia C Andrade 1, Morgana M Novaes 1, Jessica A Pessoa 1, Sergio A Mota-Rolim 1, Flávia L Osório 3, Rafael Sanches 3, Rafael G Dos Santos 3, Luís Fernando Tófoli 4, Gabriela de Oliveira Silveira 5, Mauricio Yonamine 5, Jordi Riba 6, Francisco R Santos 7, Antonio A Silva-Junior 7, João C Alchieri 8, Nicole L Galvão-Coelho 9, Bruno Lobão-Soares 9, Jaime E C Hallak 3, Emerson Arcoverde 2, João P Maia-de-Oliveira 2, Dráulio B Araújo 1
Affiliations
- PMID: 29903051
- PMCID: PMC6378413
- DOI: 10.1017/S0033291718001356
Randomized Controlled Trial
Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial
Fernanda Palhano-Fontes et al. Psychol Med. 2019 Mar.
Abstract
Background: Recent open-label trials show that psychedelics, such as ayahuasca, hold promise as fast-onset antidepressants in treatment-resistant depression.
Methods: To test the antidepressant effects of ayahuasca, we conducted a parallel-arm, double-blind randomized placebo-controlled trial in 29 patients with treatment-resistant depression. Patients received a single dose of either ayahuasca or placebo. We assessed changes in depression severity with the Montgomery-Åsberg Depression Rating Scale (MADRS) and the Hamilton Depression Rating scale at baseline, and at 1 (D1), 2 (D2), and 7 (D7) days after dosing.
Results: We observed significant antidepressant effects of ayahuasca when compared with placebo at all-time points. MADRS scores were significantly lower in the ayahuasca group compared with placebo at D1 and D2 (p = 0.04), and at D7 (p < 0.0001). Between-group effect sizes increased from D1 to D7 (D1: Cohen's d = 0.84; D2: Cohen's d = 0.84; D7: Cohen's d = 1.49). Response rates were high for both groups at D1 and D2, and significantly higher in the ayahuasca group at D7 (64% v. 27%; p = 0.04). Remission rate showed a trend toward significance at D7 (36% v. 7%, p = 0.054).
Conclusions: To our knowledge, this is the first controlled trial to test a psychedelic substance in treatment-resistant depression. Overall, this study brings new evidence supporting the safety and therapeutic value of ayahuasca, dosed within an appropriate setting, to help treat depression. This study is registered at http://clinicaltrials.gov (NCT02914769).
Keywords: Ayahuasca; HRS; MEQ; depression; psychedelics; randomized controlled trial (RCT).
Figures
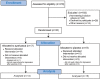
Fig. 1.
Trial profile.
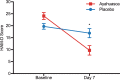
Fig. 2.
HAM-D scores at baseline and seven days after dosing. Statistical analysis shows a significant difference between ayahuasca (squares) and placebo (circles) seven days after dosing (p = 0.019). Between-group effect size is high (Cohen's d = 0.98). Values are (mean ±
s.e.m.
). HAM-D scores: mild depression (8–16), moderate (17–23), severe (⩾24).
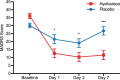
Fig. 3.
MADRS scores as a function of time. Significant differences are observed between ayahuasca (squares) and placebo (circles) at D1 (p = 0.04), D2 (p = 0.04) and D7 (p < 0.0001). Between groups effect sizes are high at all time points after dosing: D1 (Cohen's d = 0.84), D2 (Cohen's d = 0.84), and D7 (Cohen's d = 1.49). Values are (mean ±
s.e.m.
). MADRS scores: mild depression (11–19), moderate (20–34), severe (⩾35). *p < 0.05; ***p < 0.0001.
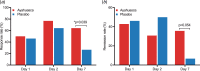
Fig. 4.
Response and remission rates as a function of time. Response (a) and remission (b) rates were high for both groups at D1 and D2. At D7, response rate was significantly higher for ayahuasca [OR 4.95 (95% CI 1.11–21.02); p = 0.04; NNT = 2.66], while remission rate showed a trend toward significance [OR 7.78 (95% CI 0.81–77.48); p = 0.054; NNT = 3.44].
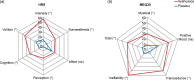
Fig. 5.
HRS subscales and MEQ30 factors during the dosing session. (a) Significantly higher scores in the ayahuasca group in five HRS subscales: perception (p < 0.0001), somaesthesia (p < 0.0001), cognition (p < 0.0001), intensity (p < 0.0001), and volition (p = 0.0003). Only affect was not significantly different between groups (p = 0.38). (b) Significantly higher MEQ30 scores in the ayahuasca group in the total MEQ30 score (p = 0.004), and three of its factors: mystical (p = 0.049), transcendence of time and space (p = 0.0008), and ineffability (p = 0.003), except for the positive mood (p = 0.32). Values are expressed as a percentage of maximum possible score.
Similar articles
- Antidepressant Effects of a Single Dose of Ayahuasca in Patients With Recurrent Depression: A SPECT Study.
Sanches RF, de Lima Osório F, Dos Santos RG, Macedo LR, Maia-de-Oliveira JP, Wichert-Ana L, de Araujo DB, Riba J, Crippa JA, Hallak JE. Sanches RF, et al. J Clin Psychopharmacol. 2016 Feb;36(1):77-81. doi: 10.1097/JCP.0000000000000436. J Clin Psychopharmacol. 2016. PMID: 26650973 Clinical Trial. - Changes in inflammatory biomarkers are related to the antidepressant effects of Ayahuasca.
Galvão-Coelho NL, de Menezes Galvão AC, de Almeida RN, Palhano-Fontes F, Campos Braga I, Lobão Soares B, Maia-de-Oliveira JP, Perkins D, Sarris J, de Araujo DB. Galvão-Coelho NL, et al. J Psychopharmacol. 2020 Oct;34(10):1125-1133. doi: 10.1177/0269881120936486. Epub 2020 Jul 10. J Psychopharmacol. 2020. PMID: 32648790 Clinical Trial. - Antidepressant effects of a single dose of ayahuasca in patients with recurrent depression: a preliminary report.
Osório Fde L, Sanches RF, Macedo LR, Santos RG, Maia-de-Oliveira JP, Wichert-Ana L, Araujo DB, Riba J, Crippa JA, Hallak JE. Osório Fde L, et al. Braz J Psychiatry. 2015 Jan-Mar;37(1):13-20. doi: 10.1590/1516-4446-2014-1496. Braz J Psychiatry. 2015. PMID: 25806551 - Novel Augmentation Strategies in Major Depression.
Martiny K. Martiny K. Dan Med J. 2017 Apr;64(4):B5338. Dan Med J. 2017. PMID: 28385173 Review. - How does ayahuasca work from a psychiatric perspective? Pros and cons of the entheogenic therapy.
Orsolini L, Chiappini S, Papanti D, Latini R, Volpe U, Fornaro M, Tomasetti C, Vellante F, De Berardis D. Orsolini L, et al. Hum Psychopharmacol. 2020 May;35(3):e2728. doi: 10.1002/hup.2728. Epub 2020 Mar 27. Hum Psychopharmacol. 2020. PMID: 32220028 Review.
Cited by
- 3,4-Methylenedioxy methamphetamine, synthetic cathinones and psychedelics: From recreational to novel psychotherapeutic drugs.
López-Arnau R, Camarasa J, Carbó ML, Nadal-Gratacós N, Puigseslloses P, Espinosa-Velasco M, Urquizu E, Escubedo E, Pubill D. López-Arnau R, et al. Front Psychiatry. 2022 Oct 3;13:990405. doi: 10.3389/fpsyt.2022.990405. eCollection 2022. Front Psychiatry. 2022. PMID: 36262632 Free PMC article. Review. - Relational Processes in Ayahuasca Groups of Palestinians and Israelis.
Roseman L, Ron Y, Saca A, Ginsberg N, Luan L, Karkabi N, Doblin R, Carhart-Harris R. Roseman L, et al. Front Pharmacol. 2021 May 19;12:607529. doi: 10.3389/fphar.2021.607529. eCollection 2021. Front Pharmacol. 2021. PMID: 34093170 Free PMC article. - The Acute Effects of the Atypical Dissociative Hallucinogen Salvinorin A on Functional Connectivity in the Human Brain.
Doss MK, May DG, Johnson MW, Clifton JM, Hedrick SL, Prisinzano TE, Griffiths RR, Barrett FS. Doss MK, et al. Sci Rep. 2020 Oct 2;10(1):16392. doi: 10.1038/s41598-020-73216-8. Sci Rep. 2020. PMID: 33009457 Free PMC article. - A rapid narrative review of the clinical evolution of psychedelic treatment in clinical trials.
Kishon R, Modlin NL, Cycowicz YM, Mourtada H, Wilson T, Williamson V, Cleare A, Rucker J. Kishon R, et al. Npj Ment Health Res. 2024 Jul 2;3(1):33. doi: 10.1038/s44184-024-00068-9. Npj Ment Health Res. 2024. PMID: 38956330 Free PMC article. Review. - The Altered States Database: Psychometric data from a systematic literature review.
Prugger J, Derdiyok E, Dinkelacker J, Costines C, Schmidt TT. Prugger J, et al. Sci Data. 2022 Nov 23;9(1):720. doi: 10.1038/s41597-022-01822-4. Sci Data. 2022. PMID: 36418335 Free PMC article.
References
- Barbosa PC, Strassman RJ, da Silveira DX, Areco K, Hoy R, Pommy J, Thoma R and Bogenschutz M (2016) Psychological and neuropsychological assessment of regular hoasca users. Comprehensive Psychiatry 71, 95–105. - PubMed
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS and Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biological Psychiatry 47, 351–354. - PubMed
- Bogenschutz MP, Forcehimes AA, Pommy JA, Wilcox CE, Barbosa P and Strassman RJ (2015) Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. Journal of Psychopharmacology 29, 289–299. - PubMed
- Bouso JC, González D, Fondevila S, Cutchet M, Fernández X, Ribeiro Barbosa PC, Alcázar-Córcoles M, Araújo WS, Barbanoj MJ, Fábregas JM and Riba J (2012) Personality, psychopathology, life attitudes and neuropsychological performance among ritual users of ayahuasca: a longitudinal study. PLoS ONE 7, e42421. - PMC - PubMed
- Bremner JD, Krystal JH, Putnam FW, Southwick SM, Marmar C, Charney DS and Mazure CM (1998) Measurement of dissociative states with the clinician-administered dissociative states scale (CADSS). Journal of Traumatic Stress 11, 125–136. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical