Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease - PubMed (original) (raw)
Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer's disease
Piotr Lewczuk et al. Alzheimers Res Ther. 2018.
Abstract
Background: A growing body of evidence suggests that the plasma concentration of the neurofilament light chain (NfL) might be considered a plasma biomarker for the screening of neurodegeneration in Alzheimer's disease (AD).
Methods: With a single molecule array method (Simoa, Quanterix), plasma NfL concentrations were measured in 99 subjects with AD at the stage of mild cognitive impairment (MCI-AD; n = 25) or at the stage of early dementia (ADD; n = 33), and in nondemented controls (n = 41); in all patients, the clinical diagnoses were in accordance with the results of the four core cerebrospinal fluid (CSF) biomarkers (amyloid β (Aβ)1-42, Aβ42/40, Tau, and pTau181), interpreted according to the Erlangen Score algorithm. The influence of preanalytical storage procedures on the NfL in plasma was tested on samples exposed to six different conditions.
Results: NfL concentrations significantly increased in the samples exposed to more than one freezing/thawing cycle, and in those stored for 5 days at room temperature or at 4 °C. Compared with the control group of nondemented subjects (22.0 ± 12.4 pg/mL), the unadjusted plasma NfL concentration was highly significantly higher in the MCI-AD group (38.1 ± 15.9 pg/mL, p < 0.005) and even further elevated in the ADD group (49.1 ± 28.4 pg/mL; p < 0.001). A significant association between NfL and age (ρ = 0.65, p < 0.001) was observed; after correcting for age, the difference in NfL concentrations between AD and controls remained significant (p = 0.044). At the cutoff value of 25.7 pg/mL, unconditional sensitivity, specificity, and accuracy were 0.84, 0.78, and 0.82, respectively. Unadjusted correlation between plasma NfL and Mini Mental State Examination (MMSE) across all patients was moderate but significant (r = -0.49, p < 0.001). We observed an overall significant correlation between plasma NfL and the CSF biomarkers, but this correlation was not observed within the diagnostic groups.
Conclusions: This study confirms increased concentrations of plasma NfL in patients with Alzheimer's disease compared with nondemented controls.
Keywords: Alzheimer’s disease; Biomarker; Neurofilament light; Plasma.
Conflict of interest statement
Ethics approval and consent to participate
The ethical committee of the University of Erlangen-Nuremberg approved the study, and all patients or their caregivers gave their written consent.
Consent for publication
Not applicable.
Competing interests
PL has received consultation and/or lecture honoraria from IBL International, Fujirebio Europe, AJ Roboscreen, and Roche. KB has served as a consultant or on advisory boards for Alzheon, BioArctic, Biogen, Eli Lilly, Fujirebio Europe, IBL International, Merck, Novartis, Pfizer, and Roche Diagnostics. KB and HZ are cofounders of Brain Biomarker Solutions in Gothenburg AB, a GU Ventures-based platform company at the University of Gothenburg. HZ has served on advisory boards of Eli Lilly and Roche Diagnostics and has received travel support from Teva. CS and JP are full-time employees of Boehringer Ingelheim. All remaining authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
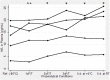
Fig. 1
Neurofilament light chain (NfL) plasma levels of samples with different preanalytical treatment. *p < 0.05, versus the reference (Ref.) sample (frozen at −80 °C and unthawed). d days, F/T freeze/thaw cycle, n.s. not significant, RT room temperature
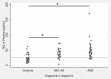
Fig. 2
Unadjusted neurofilament light chain (NfL) concentrations in the three diagnostic categories. A borderline insignificant (p = 0.11 after Scheffe correction) tendency was observed towards higher concentrations in Alzheimer’s disease dementia (ADD) compared with mild cognitive impairment Alzheimer’s disease (MCI-AD). The green horizontal line represents the cutoff at the maximized Youden index (25.7 pg/mL), leading to the unadjusted diagnostic accuracy of 82% in the setting of controls versus AD (MCI-AD and ADD). *p < 0.05
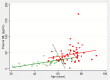
Fig. 3
Neurofilament light chain (NfL) plasma levels plotted against age in nondemented controls (green) and the AD patients (a combined group of MCI-AD and ADD subjects, red). The black dotted line represents the optimal separation of the two groups according to Fisher’s LDA
Fig. 4
a Unadjusted ROC curve in the setting of controls vs. AD. b Sensitivity (blue), specificity (brown), and accuracy (green) as functions of age. AUC area under the curve, CI confidence interval, SE standard error
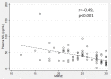
Fig. 5
Correlation between plasma neurofilament light chain (NfL) concentrations and the Mini Mental State Examination (MMSE) results
Fig. 6
Plasma neurofilament light chain (NfL) plotted against cerebrospinal fluid (CSF) Tau. a. Overall moderate, highly significant correlation between plasma NfL and CSF Tau; lacking within-group correlation between plasma NfL and CSF Tau in the controls (b), MCI-AD (c), and ADD (d)
Similar articles
- Association Between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients With Alzheimer Disease.
Mattsson N, Cullen NC, Andreasson U, Zetterberg H, Blennow K. Mattsson N, et al. JAMA Neurol. 2019 Jul 1;76(7):791-799. doi: 10.1001/jamaneurol.2019.0765. JAMA Neurol. 2019. PMID: 31009028 Free PMC article. - Predicting Longitudinal Cognitive Decline and Alzheimer's Conversion in Mild Cognitive Impairment Patients Based on Plasma Biomarkers.
Park MK, Ahn J, Kim YJ, Lee JW, Lee JC, Hwang SJ, Kim KC. Park MK, et al. Cells. 2024 Jun 22;13(13):1085. doi: 10.3390/cells13131085. Cells. 2024. PMID: 38994939 Free PMC article. - Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study.
de Wolf F, Ghanbari M, Licher S, McRae-McKee K, Gras L, Weverling GJ, Wermeling P, Sedaghat S, Ikram MK, Waziry R, Koudstaal W, Klap J, Kostense S, Hofman A, Anderson R, Goudsmit J, Ikram MA. de Wolf F, et al. Brain. 2020 Apr 1;143(4):1220-1232. doi: 10.1093/brain/awaa054. Brain. 2020. PMID: 32206776 Free PMC article. - Novel Biomarkers for Alzheimer's Disease: Plasma Neurofilament Light and Cerebrospinal Fluid.
Abukuri DN. Abukuri DN. Int J Alzheimers Dis. 2024 May 15;2024:6668159. doi: 10.1155/2024/6668159. eCollection 2024. Int J Alzheimers Dis. 2024. PMID: 38779175 Free PMC article. Review. - The potential of blood neurofilament light as a marker of neurodegeneration for Alzheimer's disease.
Jung Y, Damoiseaux JS. Jung Y, et al. Brain. 2024 Jan 4;147(1):12-25. doi: 10.1093/brain/awad267. Brain. 2024. PMID: 37540027 Free PMC article. Review.
Cited by
- Plasma neurofilament light chain as prognostic marker of cognitive decline in neurodegenerative diseases, a clinical setting study.
Götze K, Vrillon A, Dumurgier J, Indart S, Sanchez-Ortiz M, Slimi H, Raynaud-Simon A, Cognat E, Martinet M, Zetterberg H, Blennow K, Hourrègue C, Bouaziz-Amar E, Paquet C, Lilamand M. Götze K, et al. Alzheimers Res Ther. 2024 Oct 19;16(1):231. doi: 10.1186/s13195-024-01593-7. Alzheimers Res Ther. 2024. PMID: 39427171 Free PMC article. - Impact of amyloid and cardiometabolic risk factors on prognostic capacity of plasma neurofilament light chain for neurodegeneration.
Kim KY, Kim E, Lee JY; Alzheimer’s Disease Neuroimaging Initiative. Kim KY, et al. Alzheimers Res Ther. 2024 Sep 12;16(1):202. doi: 10.1186/s13195-024-01564-y. Alzheimers Res Ther. 2024. PMID: 39267169 Free PMC article. - Clinical Application of Blood Biomarkers in Neurodegenerative Diseases-Present and Future Perspectives.
Krawczuk D, Kulczyńska-Przybik A, Mroczko B. Krawczuk D, et al. Int J Mol Sci. 2024 Jul 25;25(15):8132. doi: 10.3390/ijms25158132. Int J Mol Sci. 2024. PMID: 39125699 Free PMC article. Review. - Diagnostic and predictive power of plasma proteins in Alzheimer's disease: a cross-sectional and longitudinal study in China.
Li W, Sun L, Yue L, Xiao S. Li W, et al. Sci Rep. 2024 Jul 30;14(1):17557. doi: 10.1038/s41598-024-66195-7. Sci Rep. 2024. PMID: 39080359 Free PMC article. - Plasma neurofilament light, glial fibrillary acid protein, and phosphorylated tau 181 as biomarkers for neuropsychiatric symptoms and related clinical disease progression.
Rabl M, Zullo L, Lewczuk P, Kornhuber J, Karikari TK, Blennow K, Zetterberg H, Bavato F, Quednow BB, Seifritz E, von Gunten A, Clark C, Popp J. Rabl M, et al. Alzheimers Res Ther. 2024 Jul 25;16(1):165. doi: 10.1186/s13195-024-01526-4. Alzheimers Res Ther. 2024. PMID: 39054505 Free PMC article.
References
- Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, Holtta M, Rosen C, Olsson C, Strobel G, et al. CSF and blood biomarkers for the diagnosis of Alzheimer's disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–684. doi: 10.1016/S1474-4422(16)00070-3. - DOI - PubMed
- Lewczuk P, Riederer P, O'Bryant SE, Verbeek MM, Dubois B, Visser PJ, Jellinger KA, Engelborghs S, Ramirez A, Parnetti L, et al. Cerebrospinal fluid and blood biomarkers for neurodegenerative dementias: an update of the consensus of the task force on biological markers in psychiatry of the World Federation of Societies of Biological Psychiatry. World J Biol Psychiatry. 2018;19:244–328. - PMC - PubMed
- Duits FH, Martinez-Lage P, Paquet C, Engelborghs S, Lleo A, Hausner L, Molinuevo JL, Stomrud E, Farotti L, Ramakers I, et al. Performance and complications of lumbar puncture in memory clinics: results of the multicenter lumbar puncture feasibility study. Alzheimers Dement. 2016;12:154–163. doi: 10.1016/j.jalz.2015.08.003. - DOI - PubMed
- O'Bryant SE, Mielke MM, Rissman RA, Lista S, Vanderstichele H, Zetterberg H, Lewczuk P, Posner H, Hall J, Johnson L, et al. Blood-based biomarkers in Alzheimer disease: current state of the science and a novel collaborative paradigm for advancing from discovery to clinic. Alzheimers Dement. 2017;13:45–58. doi: 10.1016/j.jalz.2016.09.014. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical