Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles - PubMed (original) (raw)
. 2018 Sep;10(5):516-532.
doi: 10.4168/aair.2018.10.5.516.
Seng Jin Choi # 2, Hyun Il Choi # 3, Jun Pyo Choi 4, Han Ki Park 5, Eun Kyoung Kim 6, Min Jeong Kim 7, Byoung Seok Moon 7, Taek Ki Min 8, Mina Rho 9, Young Joo Cho 10, Sanghwa Yang 6, Yoon Keun Kim 6, You Young Kim 11, Bok Yang Pyun 12
Affiliations
- PMID: 30088371
- PMCID: PMC6082821
- DOI: 10.4168/aair.2018.10.5.516
Lactobacillus plantarum-derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-derived Extracellular Vesicles
Min Hye Kim et al. Allergy Asthma Immunol Res. 2018 Sep.
Abstract
Purpose: The microbial environment is an important factor that contributes to the pathogenesis of atopic dermatitis (AD). Recently, it was revealed that not only bacteria itself but also extracellular vesicles (EVs) secreted from bacteria affect the allergic inflammation process. However, almost all research carried out so far was related to local microorganisms, not the systemic microbial distribution. We aimed to compare the bacterial EV composition between AD patients and healthy subjects and to experimentally find out the beneficial effect of some bacterial EV composition.
Methods: Twenty-seven AD patients and 6 healthy control subjects were enrolled. After urine and serum were obtained, EVs were prepared from samples. Metagenomic analysis of 16s ribosomal DNA extracted from the EVs was performed, and bacteria showing the greatest difference between controls and patients were identified. In vitro and in vivo therapeutic effects of significant bacterial EV were evaluated with keratinocytes and with Staphylococcus aureus-induced mouse AD models, respectively.
Results: The proportions of Lactococcus, Leuconostoc and Lactobacillus EVs were significantly higher and those of Alicyclobacillus and Propionibacterium were lower in the control group than in the AD patient group. Therefore, lactic acid bacteria were considered to be important ones that contribute to the difference between the patient and control groups. In vitro, interleukin (IL)-6 from keratinocytes and macrophages decreased and cell viability was restored with Lactobacillus plantarum-derived EV treatment prior to S. aureus EV treatment. In S. aureus-induced mouse AD models, L. plantarum-derived EV administration reduced epidermal thickening and the IL-4 level.
Conclusions: We suggested the protective role of lactic acid bacteria in AD based on metagenomic analysis. Experimental findings further suggest that L. plantarum-derived EV could help prevent skin inflammation.
Keywords: Atopic dermatitis; Lactobacillus; metagenomics; microbiome; probiotics.
Copyright © 2018 The Korean Academy of Asthma, Allergy and Clinical Immunology · The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures
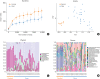
Fig. 1. (A) Alpha-diversity as determined by the Chao 1 index. (B) Beta-diversity defined by PCA. (C) Bacterial community analysis of the urine of AD patients and normal controls at the phylum level. (D) Bacterial community analysis of the urine of patients and controls at the genus level (case: AD patients, control: healthy controls).
PCA, principal component analysis; AD, atopic dermatitis.
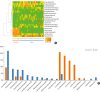
Fig. 2. (A) Differences in bacterial EVs composition in the urine of AD patients and normal controls. (B) Bacterial community analysis of the urine of AD patients and normal controls at the genus level.
EV, extracellular vesicle; AD, atopic dermatitis.
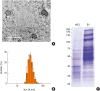
Fig. 3. Characterization of EVs from Lactobacillus plantarum. (A) TEM images of purified EVs from L. plantarum. (B) The size distribution of EVs from L. plantarum. The size distribution of EVs was measured in a diameter range of 20 to 50 nm. (C) SDS-PAGE analysis of purified proteins (WCL, EVs) from L. plantarum. Standard markers are shown on the left (kDa).
EV, extracellular vesicle; TEM, transmission electron microscopy; SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; WCL, whole cell lysate.
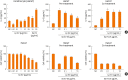
Fig. 4. (A) In vitro effects of Lactobacillus plantarum on the secretion of IL-6 from keratinocytes stimulated by Sa EV. NC was treated only with PBS, and PC was treated only with Sa EV. Pre- and co-treatment indicates that Lp EV was treated before Sa EV stimulation, or at the same time, respectively. (B) Keratinocytes cell viability assessment.
IL, interleukin; Sa EV, Staphylococcus aureus_-induced extracellular vesicle; NC, negative control; PBS, phosphate-buffered saline; PC, positive control; Lp EV, Lactobacillus plantarum_-derived extracellular vesicle. *P < 0.05 vs. NC; †_P < 0.05 vs. PC; ‡_P < 0.01 vs. PC (Mann-Whitney U test).
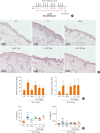
Fig. 5. In vivo effects of oral Lp EV on the AD-like skin inflammation. (A) AD mouse model using tape-stripping and Sa EV. (B) Images of H&E-stained skin. (C) Histological analysis of epidermal thickness and infiltrating eosinophil. (D) Levels of IL-4 and IL-5.
Lp EV, Lactobacillus plantarum_-derived extracellular vesicle; AD, atopic dermatitis; Sa EV, Staphylococcus aureus_-induced extracellular vesicle; H&E, hematoxylin and eosin; IL, interleukin; NC, negative control; PC, positive control. *P < 0.05 vs. NC; †_P < 0.05 vs. PC; ‡_P < 0.01 vs. PC (Mann-Whitney U test).
Similar articles
- Diagnostic Models for Atopic Dermatitis Based on Serum Microbial Extracellular Vesicle Metagenomic Analysis: A Pilot Study.
Yang J, McDowell A, Seo H, Kim S, Min TK, Jee YK, Choi Y, Park HS, Pyun BY, Kim YK. Yang J, et al. Allergy Asthma Immunol Res. 2020 Sep;12(5):792-805. doi: 10.4168/aair.2020.12.5.792. Allergy Asthma Immunol Res. 2020. PMID: 32638560 Free PMC article. - An important role of α-hemolysin in extracellular vesicles on the development of atopic dermatitis induced by Staphylococcus aureus.
Hong SW, Choi EB, Min TK, Kim JH, Kim MH, Jeon SG, Lee BJ, Gho YS, Jee YK, Pyun BY, Kim YK. Hong SW, et al. PLoS One. 2014 Jul 3;9(7):e100499. doi: 10.1371/journal.pone.0100499. eCollection 2014. PLoS One. 2014. PMID: 24992681 Free PMC article. - Extracellular vesicles derived from Staphylococcus aureus induce atopic dermatitis-like skin inflammation.
Hong SW, Kim MR, Lee EY, Kim JH, Kim YS, Jeon SG, Yang JM, Lee BJ, Pyun BY, Gho YS, Kim YK. Hong SW, et al. Allergy. 2011 Mar;66(3):351-9. doi: 10.1111/j.1398-9995.2010.02483.x. Epub 2010 Sep 9. Allergy. 2011. PMID: 20831718 Free PMC article. - State of the Art on the Role of Staphylococcus aureus Extracellular Vesicles in the Pathogenesis of Atopic Dermatitis.
Torrealba MP, Yoshikawa FSY, Aoki V, Sato MN, Orfali RL. Torrealba MP, et al. Microorganisms. 2024 Mar 6;12(3):531. doi: 10.3390/microorganisms12030531. Microorganisms. 2024. PMID: 38543582 Free PMC article. Review. - Skin microbiome dysbiosis and the role of Staphylococcus aureus in atopic dermatitis in adults and children: A narrative review.
Demessant-Flavigny AL, Connétable S, Kerob D, Moreau M, Aguilar L, Wollenberg A. Demessant-Flavigny AL, et al. J Eur Acad Dermatol Venereol. 2023 Jun;37 Suppl 5:3-17. doi: 10.1111/jdv.19125. J Eur Acad Dermatol Venereol. 2023. PMID: 37232427 Review.
Cited by
- Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-like Effects in Mice.
Choi J, Kim YK, Han PL. Choi J, et al. Exp Neurobiol. 2019 Apr;28(2):158-171. doi: 10.5607/en.2019.28.2.158. Epub 2019 Apr 30. Exp Neurobiol. 2019. PMID: 31138987 Free PMC article. - Alternatives of mesenchymal stem cell-derived exosomes as potential therapeutic platforms.
Lee S, Jung SY, Yoo D, Go D, Park JY, Lee JM, Um W. Lee S, et al. Front Bioeng Biotechnol. 2024 Sep 9;12:1478517. doi: 10.3389/fbioe.2024.1478517. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39315312 Free PMC article. Review. - Diagnostic Models for Atopic Dermatitis Based on Serum Microbial Extracellular Vesicle Metagenomic Analysis: A Pilot Study.
Yang J, McDowell A, Seo H, Kim S, Min TK, Jee YK, Choi Y, Park HS, Pyun BY, Kim YK. Yang J, et al. Allergy Asthma Immunol Res. 2020 Sep;12(5):792-805. doi: 10.4168/aair.2020.12.5.792. Allergy Asthma Immunol Res. 2020. PMID: 32638560 Free PMC article. - Extracellular membrane vesicles from Limosilactobacillus reuteri strengthen the intestinal epithelial integrity, modulate cytokine responses and antagonize activation of TRPV1.
Pang Y, Ermann Lundberg L, Mata Forsberg M, Ahl D, Bysell H, Pallin A, Sverremark-Ekström E, Karlsson R, Jonsson H, Roos S. Pang Y, et al. Front Microbiol. 2022 Nov 17;13:1032202. doi: 10.3389/fmicb.2022.1032202. eCollection 2022. Front Microbiol. 2022. PMID: 36466671 Free PMC article. - Host- and Microbiota-Derived Extracellular Vesicles, Immune Function, and Disease Development.
Macia L, Nanan R, Hosseini-Beheshti E, Grau GE. Macia L, et al. Int J Mol Sci. 2019 Dec 22;21(1):107. doi: 10.3390/ijms21010107. Int J Mol Sci. 2019. PMID: 31877909 Free PMC article. Review.
References
- Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33(Suppl 1):67–69. - PubMed
- Bieber T. Atopic dermatitis. N Engl J Med. 2008;358:1483–1494. - PubMed
- Hong SJ, Ahn KM, Lee SY, Kim KE. The prevalences of asthma and allergic diseases in Korean children. Korean J Pediatr. 2008;51:343–350.
Grants and funding
- Soonchunhyang University
- HI13C0040/Ministry of Health and Welfare
- NRF-2014R1A1A1005144/National Research Foundation of Korea
- 27301C0040/ES/NIEHS NIH HHS/United States
- NRF-2016M3A9B6901511/National Research Foundation of Korea