Measuring Artificial Sweeteners Toxicity Using a Bioluminescent Bacterial Panel - PubMed (original) (raw)
Measuring Artificial Sweeteners Toxicity Using a Bioluminescent Bacterial Panel
Dorin Harpaz et al. Molecules. 2018.
Abstract
Artificial sweeteners have become increasingly controversial due to their questionable influence on consumers' health. They are introduced in most foods and many consume this added ingredient without their knowledge. Currently, there is still no consensus regarding the health consequences of artificial sweeteners intake as they have not been fully investigated. Consumption of artificial sweeteners has been linked with adverse effects such as cancer, weight gain, metabolic disorders, type-2 diabetes and alteration of gut microbiota activity. Moreover, artificial sweeteners have been identified as emerging environmental pollutants, and can be found in receiving waters, i.e., surface waters, groundwater aquifers and drinking waters. In this study, the relative toxicity of six FDA-approved artificial sweeteners (aspartame, sucralose, saccharine, neotame, advantame and acesulfame potassium-k (ace-k)) and that of ten sport supplements containing these artificial sweeteners, were tested using genetically modified bioluminescent bacteria from E. coli. The bioluminescent bacteria, which luminesce when they detect toxicants, act as a sensing model representative of the complex microbial system. Both induced luminescent signals and bacterial growth were measured. Toxic effects were found when the bacteria were exposed to certain concentrations of the artificial sweeteners. In the bioluminescence activity assay, two toxicity response patterns were observed, namely, the induction and inhibition of the bioluminescent signal. An inhibition response pattern may be observed in the response of sucralose in all the tested strains: TV1061 (MLIC = 1 mg/mL), DPD2544 (MLIC = 50 mg/mL) and DPD2794 (MLIC = 100 mg/mL). It is also observed in neotame in the DPD2544 (MLIC = 2 mg/mL) strain. On the other hand, the induction response pattern may be observed in its response in saccharin in TV1061 (MLIndC = 5 mg/mL) and DPD2794 (MLIndC = 5 mg/mL) strains, aspartame in DPD2794 (MLIndC = 4 mg/mL) strain, and ace-k in DPD2794 (MLIndC = 10 mg/mL) strain. The results of this study may help in understanding the relative toxicity of artificial sweeteners on E. coli, a sensing model representative of the gut bacteria. Furthermore, the tested bioluminescent bacterial panel can potentially be used for detecting artificial sweeteners in the environment, using a specific mode-of-action pattern.
Keywords: artificial sweeteners; bioluminescent bacteria; environmental pollutants; gut microbiota; sport supplements; toxic effect.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
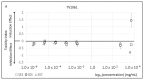
Figure 1
Artificial sweeteners toxicity. The toxicity index of different artificial sweeteners on the three tested bioluminescent bacteria strains: (A) TV1061; (B) DPD2544; (C) DPD2794. A strong induction response pattern may be observed in the response of the TV1061 strain to saccharin and DPD2794 strain to aspartame and saccharin. In addition, a strong inhibition response pattern may be observed in the response of the TV1061 strain to sucralose.
Figure 2
Sport supplements’ toxicity. Toxicity index of different sport supplements on the three tested bioluminescent bacteria strains: (A) TV1061; (B) DPD2544; (C) DPD2794.
Figure 2
Sport supplements’ toxicity. Toxicity index of different sport supplements on the three tested bioluminescent bacteria strains: (A) TV1061; (B) DPD2544; (C) DPD2794.
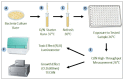
Figure 3
Experimental process. (A) each bacteria strain tested was striked on an agar plate containing Kanamycin, and incubated overnight at 37 °C; (B) a starter was grown from a single colony from the striked plate, and incubated overnight at 37 °C in a shaking incubator; (C) the starter was refreshed by adding 200 μL of the overnight culture into 10 mL of fresh LB, and then grown for 3–4 h at 30 °C in a non-shaking incubator; (D) the bacteria strains were then exposed to the different samples of different concentrations in a high-throughput measurement using a 96-well plate; (E,F) the toxicity (Relative Light Unit (RLU)) and growth (O.D. 600 nm) signals were measured continuously during the 16 h incubation at 26 °C, in the Luminometer and TECAN reader, respectively.
Similar articles
- Inhibitory Effects of Artificial Sweeteners on Bacterial Quorum Sensing.
Markus V, Share O, Shagan M, Halpern B, Bar T, Kramarsky-Winter E, Teralı K, Özer N, Marks RS, Kushmaro A, Golberg K. Markus V, et al. Int J Mol Sci. 2021 Sep 13;22(18):9863. doi: 10.3390/ijms22189863. Int J Mol Sci. 2021. PMID: 34576027 Free PMC article. - Artificial sweeteners: safe or unsafe?
Qurrat-ul-Ain, Khan SA. Qurrat-ul-Ain, et al. J Pak Med Assoc. 2015 Feb;65(2):225-7. J Pak Med Assoc. 2015. PMID: 25842566 Review. - Artificial sweeteners--do they bear a carcinogenic risk?
Weihrauch MR, Diehl V. Weihrauch MR, et al. Ann Oncol. 2004 Oct;15(10):1460-5. doi: 10.1093/annonc/mdh256. Ann Oncol. 2004. PMID: 15367404 Review. - Fate of artificial sweeteners in wastewater treatment plants in New York State, U.S.A.
Subedi B, Kannan K. Subedi B, et al. Environ Sci Technol. 2014 Dec 2;48(23):13668-74. doi: 10.1021/es504769c. Epub 2014 Nov 14. Environ Sci Technol. 2014. PMID: 25365516
Cited by
- Potential Effects of Sucralose and Saccharin on Gut Microbiota: A Review.
Del Pozo S, Gómez-Martínez S, Díaz LE, Nova E, Urrialde R, Marcos A. Del Pozo S, et al. Nutrients. 2022 Apr 18;14(8):1682. doi: 10.3390/nu14081682. Nutrients. 2022. PMID: 35458244 Free PMC article. Review. - Inhibitory Effects of Artificial Sweeteners on Bacterial Quorum Sensing.
Markus V, Share O, Shagan M, Halpern B, Bar T, Kramarsky-Winter E, Teralı K, Özer N, Marks RS, Kushmaro A, Golberg K. Markus V, et al. Int J Mol Sci. 2021 Sep 13;22(18):9863. doi: 10.3390/ijms22189863. Int J Mol Sci. 2021. PMID: 34576027 Free PMC article. - High Concentrations of Aspartame Induce Pro-Angiogenic Effects in Ovo and Cytotoxic Effects in HT-29 Human Colorectal Carcinoma Cells.
Maghiari AL, Coricovac D, Pinzaru IA, Macașoi IG, Marcovici I, Simu S, Navolan D, Dehelean C. Maghiari AL, et al. Nutrients. 2020 Nov 24;12(12):3600. doi: 10.3390/nu12123600. Nutrients. 2020. PMID: 33255204 Free PMC article. - Aspartame, acesulfame K and sucralose- influence on the metabolism of Escherichia coli.
Shahriar S, Ahsan T, Khan A, Akhteruzzaman S, Shehreen S, Sajib AA. Shahriar S, et al. Metabol Open. 2020 Dec 4;8:100072. doi: 10.1016/j.metop.2020.100072. eCollection 2020 Dec. Metabol Open. 2020. PMID: 33336183 Free PMC article. - Complexes of Cu-Polysaccharide of a Marine Red Microalga Produce Spikes with Antimicrobial Activity.
Yehuda N, Gheber LA, Kushmaro A, Mails Arad S. Yehuda N, et al. Mar Drugs. 2022 Dec 19;20(12):787. doi: 10.3390/md20120787. Mar Drugs. 2022. PMID: 36547934 Free PMC article.
References
- FDA, High-Intensity Sweeteners U.S. Food and Drug Administration. [(accessed on 19 May 2014)]; Available online: https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingre....
- EFSA, Sugars and Sweeteners European Food Safety Authority. [(accessed on 30 January 2018)]; Available online: https://ec.europa.eu/jrc/en/health-knowledge-gateway/promotion-preventio....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous