Gut Mechanisms Linking Intestinal Sweet Sensing to Glycemic Control - PubMed (original) (raw)
Gut Mechanisms Linking Intestinal Sweet Sensing to Glycemic Control
Denise Kreuch et al. Front Endocrinol (Lausanne). 2018.
Abstract
Sensing nutrients within the gastrointestinal tract engages the enteroendocrine cell system to signal within the mucosa, to intrinsic and extrinsic nerve pathways, and the circulation. This signaling provides powerful feedback from the intestine to slow the rate of gastric emptying, limit postprandial glycemic excursions, and induce satiation. This review focuses on the intestinal sensing of sweet stimuli (including low-calorie sweeteners), which engage similar G-protein-coupled receptors (GPCRs) to the sweet taste receptors (STRs) of the tongue. It explores the enteroendocrine cell signals deployed upon STR activation that act within and outside the gastrointestinal tract, with a focus on the role of this distinctive pathway in regulating glucose transport function via absorptive enterocytes, and the associated impact on postprandial glycemic responses in animals and humans. The emerging role of diet, including low-calorie sweeteners, in modulating the composition of the gut microbiome and how this may impact glycemic responses of the host, is also discussed, as is recent evidence of a causal role of diet-induced dysbiosis in influencing the gut-brain axis to alter gastric emptying and insulin release. Full knowledge of intestinal STR signaling in humans, and its capacity to engage host and/or microbiome mechanisms that modify glycemic control, holds the potential for improved prevention and management of type 2 diabetes.
Keywords: L-cells; SGLT-1; glucose transport; glycemic control; intestinal sweet taste receptors; type 2 diabetes mellitus.
Figures
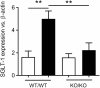
Figure 1
STR-dependence of SGLT-1 expression in mice. Increased jejunal expression of SGLT-1 mRNA in 10 week-old control (WT/WT) mice gavaged for 4 days with sucralose (black bars) compared to water (white bars), and to mice homozygous for both Tas1r2 and Tas1r3 genes (KO/KO). Breeding pairs of mice homozygous for the Tas1r2 or Tas1r3 gene (129X1/SvJ mice backcrossed for at least 3 generations with C57BL/6 mice) were provided by Prof Charles Zuker (University of California, San Diego, USA). Mice homogenous for each gene were then paired to produce mice heterozygous for Tas1r2 and Tas1r3. These mice, in turn, were paired to generate mice heterozygous, homozygous, and wild-type for both genes. From these mice, double homozygous (KO/KO) and wild-type littermate controls (WT/WT) were the subject of gavage experiments. Ten-week old male mice (N = 5 per group) maintained under standard housing and diet conditions in the SA Pathology Animal Care Facility were gavaged twice daily with 100 mg sucralose (Redox Chemicals, Minto, NSW Australia) in 200 μL water, or 200 μL water, at 0800 and 1800 over 4 days. These mice were fasted overnight then humanely killed at 0800, total RNA extracted from the jejunal mucosa, and real-time RT-PCR performed using primer assays for SGLT-1 (QT00112679) and β-actin (QT01136772, Qiagen, Sydney, NSW Australia) relative to expression of β-actin, as described (49); SGLT-1 expression was compared between groups and gavage regime by analysis of variance (ANOVA), adjusted for multiple comparisons by Holm-Sidak's correction (GraphPad Prism 7.02, San Diego, CA, USA). This experiment was approved and performed in accordance with guidelines of the Animal Ethics Committees of The University of Adelaide and SA Pathology (Adelaide, Australia). Data is shown as Mean ± SEM; **P < 0.01. We thank Prof Charles Zuker for generously supplying the homozygous Tas1r2 and Tas1r3 mice.
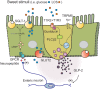
Figure 2
Model of intestinal sweet taste sensing and signaling effectors. Sweet stimuli, including LCS, bind to STR comprised of a heterodimer of G-protein coupled receptors T1R2 and T1R3. Upon receptor binding an intracellular signaling cascade is activated, initiated by dissociation of G-protein gustducin into Gα and Gβγ subunits and activation of phospholipase C β2 (PLCβ2); intracellular Ca2+ is then released from inositol 1,4,5-trisphosphate-sensitive stores, leading to opening of the melastatin type-5 transient receptor potential cation channel (TRPM5) to sodium influx. Increases in intracellular Na+ and Ca2+ then depolarize the basolateral membrane, to facilitate release of peptide hormones such as GLP-2. GLP-2 may then trigger an enteric neuron pathway to release an unknown neuropeptide at nearby absorptive enterocytes leading to adenylate cyclase-dependent stablilization of the 3' end of SGLT-1 mRNA (to increase half-life), and SGLT-1 translation and insertion into the apical brush border membrane.
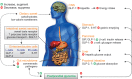
Figure 3
Gastrointestinal factors influencing glycemic control. Dietary sweet stimuli can activate STR in the proximal intestine facilitating the enteroendocrine cell release of the incretin peptides GIP from K-cells and GLP-1 from L-cells, as well as 5-HT from EC-cells; substrates of SGLT-1 (glucose, galactose) also trigger GIP and GLP-2 release. GIP and GLP-1 stimulate glucose-dependent insulin release, to increase glucose disposal; GLP-1 and 5-HT also slow the rate of gastric emptying via vagus nerve signals (not shown) while GLP-1 inhibits pancreatic glucagon release, leading to reduced hepatic glucose output. GLP-2 co-released from L-cells acts to increase intestinal glucose absorption via an increase in the capacity for SGLT-1-based glucose transport. Dietary sweet stimuli can also alter the composition of the gut microbiome in favor of colonization of gut pathogens over fermentative gut commensals, which can affect energy harvest, and disrupt microbiome signaling to the host and glycemic control. Together these influences can disrupt the homeostatic balance between glucose-evoked gut hormone release, glucose absorption, and microbiome composition, leading to dysglycemia which would potentially be harmful in the setting of type 2 diabetes. In addition, complex carbohydrates (oligosaccharides) may contribute to these processes via a yet to be identified polycose taste receptor.
Similar articles
- Role of Intestinal Bitter Sensing in Enteroendocrine Hormone Secretion and Metabolic Control.
Xie C, Wang X, Young RL, Horowitz M, Rayner CK, Wu T. Xie C, et al. Front Endocrinol (Lausanne). 2018 Sep 27;9:576. doi: 10.3389/fendo.2018.00576. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30319553 Free PMC article. Review. - Metabolic control via nutrient-sensing mechanisms: role of taste receptors and the gut-brain neuroendocrine axis.
Raka F, Farr S, Kelly J, Stoianov A, Adeli K. Raka F, et al. Am J Physiol Endocrinol Metab. 2019 Oct 1;317(4):E559-E572. doi: 10.1152/ajpendo.00036.2019. Epub 2019 Jul 16. Am J Physiol Endocrinol Metab. 2019. PMID: 31310579 Review. - Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells.
Dyer J, Salmon KS, Zibrik L, Shirazi-Beechey SP. Dyer J, et al. Biochem Soc Trans. 2005 Feb;33(Pt 1):302-5. doi: 10.1042/BST0330302. Biochem Soc Trans. 2005. PMID: 15667333 - Gut sweet taste receptors and their role in metabolism.
Meyer-Gerspach AC, Wölnerhanssen B, Beglinger C. Meyer-Gerspach AC, et al. Front Horm Res. 2014;42:123-33. doi: 10.1159/000358321. Epub 2014 Apr 7. Front Horm Res. 2014. PMID: 24732930 Review. - Sweet taste receptor expression in ruminant intestine and its activation by artificial sweeteners to regulate glucose absorption.
Moran AW, Al-Rammahi M, Zhang C, Bravo D, Calsamiglia S, Shirazi-Beechey SP. Moran AW, et al. J Dairy Sci. 2014;97(8):4955-72. doi: 10.3168/jds.2014-8004. Epub 2014 Jun 2. J Dairy Sci. 2014. PMID: 24881785
Cited by
- The Effects of Artificial Sweeteners on Intestinal Nutrient-Sensing Receptors: Dr. Jekyll or Mr. Hyde?
Posta E, Fekete I, Gyarmati E, Stündl L, Zold E, Barta Z. Posta E, et al. Life (Basel). 2023 Dec 20;14(1):10. doi: 10.3390/life14010010. Life (Basel). 2023. PMID: 38276259 Free PMC article. Review. - The glucagon-like peptide-1 (GLP-1) analogue semaglutide reduces alcohol drinking and modulates central GABA neurotransmission.
Chuong V, Farokhnia M, Khom S, Pince CL, Elvig SK, Vlkolinsky R, Marchette RC, Koob GF, Roberto M, Vendruscolo LF, Leggio L. Chuong V, et al. JCI Insight. 2023 Jun 22;8(12):e170671. doi: 10.1172/jci.insight.170671. JCI Insight. 2023. PMID: 37192005 Free PMC article. - Gastrointestinal Sensing of Meal-Related Signals in Humans, and Dysregulations in Eating-Related Disorders.
Hajishafiee M, Bitarafan V, Feinle-Bisset C. Hajishafiee M, et al. Nutrients. 2019 Jun 8;11(6):1298. doi: 10.3390/nu11061298. Nutrients. 2019. PMID: 31181734 Free PMC article. Review. - Early-life influences of low-calorie sweetener consumption on sugar taste.
Chometton S, Tsan L, Hayes AMR, Kanoski SE, Schier LA. Chometton S, et al. Physiol Behav. 2023 May 15;264:114133. doi: 10.1016/j.physbeh.2023.114133. Epub 2023 Feb 18. Physiol Behav. 2023. PMID: 36801464 Free PMC article. Review. - Mechanisms controlling hormone secretion in human gut and its relevance to metabolism.
Martin AM, Sun EW, Keating DJ. Martin AM, et al. J Endocrinol. 2019 Nov 29;244(1):R1-R15. doi: 10.1530/JOE-19-0399. J Endocrinol. 2019. PMID: 31751295 Free PMC article. Review.
References
- Sjolund K, Sanden G, Hakanson R, Sundler F. Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology (1983) 85:1120–30. - PubMed
LinkOut - more resources
Full Text Sources