Mass Spectrometry Amyloid Typing Is Reproducible across Multiple Organ Sites - PubMed (original) (raw)
Mass Spectrometry Amyloid Typing Is Reproducible across Multiple Organ Sites
Dusan Holub et al. Biomed Res Int. 2019.
Abstract
We have determined patient's amyloid subtype through immunohistochemical and proteomic analyses of formalin-fixed, paraffin-embedded (FFPE) tissue samples from two affected organs per patient. Amyloid typing, via immunohistochemistry (IHC) and laser microdissection followed by the combination of liquid chromatography with mass spectrometry (LMD-LC-MS), was performed using tissue samples of the human heart, liver, kidney, tongue, and small intestine from 11 patients, and the results were compared with clinical data. LMD-LC-MS correctly typed AL amyloidosis in all 22 FFPE tissue samples despite tissue origin. In contrast, IHC was successful only in the analysis of eight FFPE tissue samples with differences between the examined organs. In the majority of LMD-LC-MS typed samples, the level of IHC staining intensity for transthyretin and serum amyloid A was the same as that for Ig κ and Ig λ antibodies, suggesting low Ig κ or Ig λ antibodies reactivity and the additional antibody clones were essential for correct typing. Both methods used in the study were found to be suitable for amyloid typing, although LMD-LC-MS yielded more promising results than IHC.
Figures
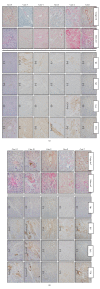
Figure 1
Immunohistochemical typing of amyloid in myocardial tissue by a basic panel of antibodies. The confirmation of amyloid deposition was done via Congo red and Sirius red staining, the amyloid typing via IHC analysis (right panels). The tissue sample of case 1 failed during IHC staining with AL κ, AL λ, SAA, and TTR antibodies. The examined tissues of cases 2 and 3 had a false positive reaction with SAA and/or TTR antibodies, weak and/or negative reaction with AL κ antibody, respectively. In cases 4, 7, 10, and 11 examined tissue had a positive reaction with more than one antibody which is classified as no immunospecific staining (NS). Amyloid fibril protein (AL κ) was typed correctly in case 5. Amyloid fibril protein (AL λ) was typed correctly in cases 6, 8, and 9. IHC staining intensity was classified as negative (-), weak (+), moderate (++), and strong (+++). The amyloid subtype was determined based on the strongest IHC reaction.
Similar articles
- [Amyloid typing from formalin-fixed paraffin-embedded tissues using LMD-LC-MS/MS system].
Tasaki M, Obayashi K, Ueda M, Ando Y. Tasaki M, et al. Rinsho Byori. 2014 Mar;62(3):291-6. Rinsho Byori. 2014. PMID: 24800507 Review. Japanese. - [Classification of amyloidosis by laser micro-dissection and mass spectrometry based proteomic analysis].
Shen K, Sun W, Sun J, Sun W, Zhong D, Cao X, Zhou D, Li J. Shen K, et al. Zhonghua Xue Ye Xue Za Zhi. 2015 Feb;36(2):99-102. doi: 10.3760/cma.j.issn.0253-2727.2015.02.003. Zhonghua Xue Ye Xue Za Zhi. 2015. PMID: 25778882 Free PMC article. Chinese. - Autopsy case with concurrent transthyretin and immunoglobulin amyloidosis.
Shintani-Domoto Y, Ishino K, Naiki H, Sakatani T, Ohashi R. Shintani-Domoto Y, et al. Pathol Int. 2022 Jan;72(1):65-71. doi: 10.1111/pin.13179. Epub 2021 Oct 12. Pathol Int. 2022. PMID: 34637570 - Pathology and Proteomics-Based Diagnosis of Localized Light-Chain Amyloidosis in Dogs and Cats.
Kadota A, Iwaide S, Miyazaki S, Mitsui I, Machida N, Murakami T. Kadota A, et al. Vet Pathol. 2020 Sep;57(5):658-665. doi: 10.1177/0300985820934113. Epub 2020 Jun 17. Vet Pathol. 2020. PMID: 32880234 - [New advances in the subtyping of systemic amyloidosis].
Sun WY, Li J. Sun WY, et al. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014 Feb;22(1):259-62. doi: 10.7534/j.issn.1009-2137.2014.01.052. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2014. PMID: 24598691 Review. Chinese.
Cited by
- Tandem Mass Spectrometry-Based Amyloid Typing Using Manual Microdissection and Open-Source Data Processing.
Phipps WS, Smith KD, Yang HY, Henderson CM, Pflaum H, Lerch ML, Fondrie WE, Emrick MA, Wu CC, MacCoss MJ, Noble WS, Hoofnagle AN. Phipps WS, et al. Am J Clin Pathol. 2022 May 4;157(5):748-757. doi: 10.1093/ajcp/aqab185. Am J Clin Pathol. 2022. PMID: 35512256 Free PMC article. - Dissecting the Molecular Features of Systemic Light Chain (AL) Amyloidosis: Contributions from Proteomics.
Rognoni P, Mazzini G, Caminito S, Palladini G, Lavatelli F. Rognoni P, et al. Medicina (Kaunas). 2021 Aug 31;57(9):916. doi: 10.3390/medicina57090916. Medicina (Kaunas). 2021. PMID: 34577839 Free PMC article. Review. - The Clinical Impact of Proteomics in Amyloid Typing.
Hill MM, Dasari S, Mollee P, Merlini G, Costello CE, Hazenberg BPC, Grogan M, Dispenzieri A, Gertz MA, Kourelis T, McPhail ED. Hill MM, et al. Mayo Clin Proc. 2021 May;96(5):1122-1127. doi: 10.1016/j.mayocp.2020.12.002. Epub 2021 Apr 9. Mayo Clin Proc. 2021. PMID: 33840526 Free PMC article. No abstract available. - Nerve biopsy: Current indications and decision tools.
Nathani D, Spies J, Barnett MH, Pollard J, Wang MX, Sommer C, Kiernan MC. Nathani D, et al. Muscle Nerve. 2021 Aug;64(2):125-139. doi: 10.1002/mus.27201. Epub 2021 Feb 25. Muscle Nerve. 2021. PMID: 33629393 Free PMC article. Review. - Quantitative Proteomic Analysis Using Formalin-Fixed, Paraffin-Embedded (FFPE) Human Cardiac Tissue.
Azimzadeh O, Atkinson MJ, Tapio S. Azimzadeh O, et al. Methods Mol Biol. 2021;2261:525-533. doi: 10.1007/978-1-0716-1186-9_33. Methods Mol Biol. 2021. PMID: 33421012
References
- Hawkins P. N. Hereditary systemic amyloidosis with renal involvement. Journal of Nephrology. 2003;16:443–448. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials