Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method - PubMed (original) (raw)
Isolation and characterization of human gingiva-derived mesenchymal stem cells using limiting dilution method
Lingqian Du et al. J Dent Sci. 2016 Sep.
Abstract
Background/purpose: Gingiva-derived mesenchymal stem cells (GMSCs) are attractive alternative MSC sources because of their relative abundance of sources and ease of accessibility. However, the isolation method for harboring GMSCs remains under discussion. The aim of the study was to isolate and explore in vitro characterization of human GMSCs, and compare stem cell properties with bulk-cultured gingival fibroblasts (GFs).
Materials and methods: GMSCs were isolated with limiting dilution method. Tissue-matched bulk-cultured GFs and GMSCs were evaluated in terms of their colony-forming abilities, population doubling capacities, cell surface epitopes, and multilineage differentiation potentials.
Results: GMSCs showed a significantly higher number of colony-forming units-fibroblast (P < 0.001) than bulk-cultured GFs, while the population doubling capacity of GMSCs reduced. Both types of cells were uniformly positive for MSC-associated makers CD44, CD73, CD90, CD105, and CD166, and were negative for hematopoietic markers CD14, CD34, and CD45. The only distinct marker was STRO-1, which was more highly expressed in GMSCs (13.4%) than in bulk-cultured GFs (0.02%). Upon induction, GMSCs displayed the capacity to undergo osteogenic, adipogenic, and chondrogenic differentiation. Real-time polymerase chain reaction showed related gene levels were significantly upregulated (P < 0.001). By contrast, bulk-cultured GFs lacked the capacity to undergo multilineage differentiation, and related gene levels showed no significant difference when compared with control groups.
Conclusion: The data validate the effectiveness of limiting dilution method for GMSCs isolation. GMSCs, in contrast to bulk-cultured GFs, harbor stem cell characteristics and can act as alternative cell sources for tissue engineering.
Keywords: differentiation; epitopes; gingiva; stem cells.
Figures
Figure 1
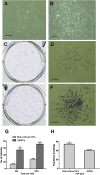
Characterization of human bulk-cultured gingival fibroblasts (GFs) and gingiva-derived mesenchymal stem cells (GMSCs). (A) Cultured primary cells derived from human gingival tissue exhibited typical fibroblast-like morphology. Scale bar: 500 μm; (B) MSC-like colonies grown for 10–14 days culture. Scale bar: 500 μm; (C) single colonies formed after bulk-cultured GFs; (D) cell clusters derived from a single colony of bulk-cultured GFs; (E) GMSCs were plated at low density and cultured for 2 weeks; (F) GMSCs stained with 0.1% toluidine blue. Scale bar: 500 μm; (G) comparison of the number of colony-forming unit-fibroblasts derived from bulk-cultured GFs and GMSCs at 500 cells and 1000 cells plated per well (data were obtained from 6 individual experiments); (H) population doublings of bulk-cultured GFs and GMSCs following successive cell passages until cellular senescence was reached (data represent 15 GF lines and 15 clonal GMSC lines derived from 3 gingival tissues). Data are presented as mean ± standard deviation. * P < 0.001.
Figure 2
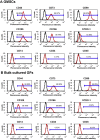
(A) Flow cytometry analyses of the expression of cell surface markers related to mesenchymal stem cells (CD44, CD73, CD90, CD105, CD166, and STRO-1); (B) flow cytometry analyses of the expression of cell surface markers related to hematopoietic stem cells (CD14, CD34, and CD45) (red line). Isotype controls 1B5 (immunoglobulin G-1) and 1D4.5 (immunoglobulin G-2a) are represented by a black line. GF = gingival fibroblasts; GMSCs = gingiva-derived mesenchymal stem cells.
Figure 3
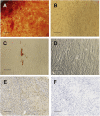
Representative imagines demonstrated multilineage differentiation of bulk-cultured gingival fibroblasts (GFs) and gingiva-derived mesenchymal stem cells (GMSCs). (A) Alizarin Red staining of the osteogenically stimulated GMSCs; (B) Alizarin Red staining of the osteogenically stimulated bulk-cultured GFs (scale bar: 200 μm); (C) Oil Red O staining of the adipogenically stimulated GMSCs; (D) Oil Red O staining of the adipogenically stimulated bulk-cultured GFs (scale bar: 50 μm); (E) immunohistochemical staining with anticollagen type II antibody of the chondrogenically stimulated GMSCs; (F) immunohistochemical staining with anticollagen type II antibody of the chondrogenically stimulated bulk-cultured GFs (scale bar: 100 μm).
Figure 4
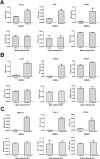
Real time-polymerase chain reaction analyses of osteogenic, adipogenic, and chondrogenic-related gene expression after 28 days. (A) Representative gene expression levels of osteogenic markers RUNX2/CBFA1, OPN, and BSP2 in gingiva-derived mesenchymal stem cells (GMSCs) and bulk-cultured gingival fibroblasts (GFs) in nonosteogenic (control) and osteogenic medium; (B) representative gene expression levels of adipogenic markers leptin, PPARγ2, and adipsin in GMSCs and bulk-cultured GFs in nonadipogenic (control) and adipogenic medium; (C) representative gene expression levels of chondrogenic markers aggrecan, COL-II, and SOX9 in GMSCs and bulk-cultured GFs in nonchondrogenic (control) and chondrogenic medium. Data are presented as mean ± standard error. * P < 0.001.
Similar articles
- Isolation and characterization of human mesenchymal stem cells from gingival connective tissue.
Jin SH, Lee JE, Yun JH, Kim I, Ko Y, Park JB. Jin SH, et al. J Periodontal Res. 2015 Aug;50(4):461-7. doi: 10.1111/jre.12228. Epub 2014 Sep 17. J Periodontal Res. 2015. PMID: 25229614 - A comparative study on immunophenotypic characterization and osteogenic differentiation of human mesenchymal stromal cells derived from periodontal ligament and gingiva.
Abedian Z, Jenabian N, Moghadamnia AA, Zabihi E, Pourbagher R, Hossein-Nataj H, Mohamadnia-Afrouzi M. Abedian Z, et al. J Periodontol. 2020 Sep;91(9):1194-1202. doi: 10.1002/JPER.19-0535. Epub 2020 Jul 17. J Periodontol. 2020. PMID: 31960428 - Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions.
Yang H, Gao LN, An Y, Hu CH, Jin F, Zhou J, Jin Y, Chen FM. Yang H, et al. Biomaterials. 2013 Sep;34(29):7033-47. doi: 10.1016/j.biomaterials.2013.05.025. Epub 2013 Jun 13. Biomaterials. 2013. PMID: 23768902 - Characteristics and Tissue Regeneration Properties of Gingiva-Derived Mesenchymal Stem Cells.
Zhao N, Wu Z, Qin L, Guo Z, Li D. Zhao N, et al. Crit Rev Eukaryot Gene Expr. 2015;25(2):135-44. doi: 10.1615/critreveukaryotgeneexpr.2015012539. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080607 Review. - Gingiva-Derived Mesenchymal Stem Cells: Potential Application in Tissue Engineering and Regenerative Medicine - A Comprehensive Review.
Kim D, Lee AE, Xu Q, Zhang Q, Le AD. Kim D, et al. Front Immunol. 2021 Apr 16;12:667221. doi: 10.3389/fimmu.2021.667221. eCollection 2021. Front Immunol. 2021. PMID: 33936109 Free PMC article. Review.
Cited by
- Dental Pulp Stem Cell-Derived Secretome and Its Regenerative Potential.
Bar JK, Lis-Nawara A, Grelewski PG. Bar JK, et al. Int J Mol Sci. 2021 Nov 6;22(21):12018. doi: 10.3390/ijms222112018. Int J Mol Sci. 2021. PMID: 34769446 Free PMC article. Review. - Therapeutic potential of dental stem cells.
Chalisserry EP, Nam SY, Park SH, Anil S. Chalisserry EP, et al. J Tissue Eng. 2017 May 23;8:2041731417702531. doi: 10.1177/2041731417702531. eCollection 2017 Jan-Dec. J Tissue Eng. 2017. PMID: 28616151 Free PMC article. Review. - Toll-Like Receptors and Dental Mesenchymal Stromal Cells.
Andrukhov O. Andrukhov O. Front Oral Health. 2021 Apr 16;2:648901. doi: 10.3389/froh.2021.648901. eCollection 2021. Front Oral Health. 2021. PMID: 35048000 Free PMC article. Review. - Rho-kinase inhibitor Y-27632 facilitates the proliferation, migration and pluripotency of human periodontal ligament stem cells.
Wang T, Kang W, Du L, Ge S. Wang T, et al. J Cell Mol Med. 2017 Nov;21(11):3100-3112. doi: 10.1111/jcmm.13222. Epub 2017 Jun 29. J Cell Mol Med. 2017. PMID: 28661039 Free PMC article. - Sinking Our Teeth in Getting Dental Stem Cells to Clinics for Bone Regeneration.
Shoushrah SH, Transfeld JL, Tonk CH, Büchner D, Witzleben S, Sieber MA, Schulze M, Tobiasch E. Shoushrah SH, et al. Int J Mol Sci. 2021 Jun 15;22(12):6387. doi: 10.3390/ijms22126387. Int J Mol Sci. 2021. PMID: 34203719 Free PMC article. Review.
References
- Halleux C., Sottile V., Gasser J.A., Seuwen K. Multi-lineage potential of human mesenchymal stem cells following clonal expansion. J Musculoskelet Neuronal Interact. 2001;2:71–76. - PubMed
- Lee R.H., Kim B., Choi I. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell Physiol Biochem. 2004;14:311–324. - PubMed
- Lee O.K., Kuo T.K., Chen W.M., Lee K.D., Hsieh S.L., Chen T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood. 2004;103:1669–1675. - PubMed
- Yen B.L., Huang H.I., Chien C.C. Isolation of multipotent cells from human term placenta. Stem Cells. 2005;23:3–9. - PubMed
- Caplan A.I. Mesenchymal stem cells. J Orthop Res. 1991;9:641–650. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous