Deregulation of SATB2 in carcinogenesis with emphasis on miRNA-mediated control - PubMed (original) (raw)
Review
Deregulation of SATB2 in carcinogenesis with emphasis on miRNA-mediated control
Qiao Yi Chen et al. Carcinogenesis. 2019.
Abstract
The special AT-rich DNA binding protein (SATB2) is a nuclear matrix-associated protein and an important transcription factor for biological development, gene regulation and chromatin remodeling. Aberrant regulation of SATB2 has been found to highly correlate with various types of cancers including lung, colon, prostate, breast, gastric and liver. Recent studies have revealed that a subset of small non-coding RNAs, termed microRNAs (miRNAs), are important regulators of SATB2 function. As post-transcriptional regulators, miRNAs have been found to have fundament importance maintaining normal cellular development. Evidence suggests that multiple miRNAs, including miR-31, miR-34, miR-182, miR-211, miR-599, are capable of regulating SATB2 in cancers of the lung, liver, colon and breast. This review examines the molecular functions of SATB2 and miRNAs in the text of cancer development and potential strategies for cancer therapy with a focus on systemic miRNA delivery.
© The Author(s) 2019. Published by Oxford University Press. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures
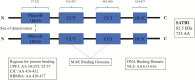
Figure 1.
Linearized SATB2 protein structure. The linearized protein structure demonstrates site of dimerization and binding domains.
Figure 2.
SATB2-induced chromatin looping provides topological organization and enhanced gene expression. The figure shows how SATB2 binds to the MAR sequence along a gene and facilitates chromatin looping, which brings distal genomic loci into close spatial proximity.
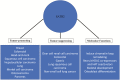
Figure 3.
Dual role of SATB2 in carcinogenesis and its molecular functions. The figure summarizes the molecular functions of SATB2 as well as its oncogenic and tumor-suppressive roles under different cancer contexts.
Figure 4.
Illustration of miRNA binding site on SATB2 mRNA. The illustration depicts SATB2 mRNA and miRNA interaction. SATB2 has multiple binding sites for miRNAs, which are exclusively found in the 3′-UTR.
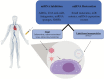
Figure 5.
Illustration of miRNA delivery. miRNA delivery can be used to either inhibit target mRNAs or restore miRNA supply. The figure illustrates two prominent ways, viral and lipid-based nanoparticles, of systemic miRNA delivery in the human body.
Similar articles
- The role of miR-31 and its target gene SATB2 in cancer-associated fibroblasts.
Aprelikova O, Yu X, Palla J, Wei BR, John S, Yi M, Stephens R, Simpson RM, Risinger JI, Jazaeri A, Niederhuber J. Aprelikova O, et al. Cell Cycle. 2010 Nov 1;9(21):4387-98. doi: 10.4161/cc.9.21.13674. Epub 2010 Nov 17. Cell Cycle. 2010. PMID: 20980827 Free PMC article. - Dental Follicle Cells Participate in Tooth Eruption via the RUNX2-MiR-31-SATB2 Loop.
Ge J, Guo S, Fu Y, Zhou P, Zhang P, Du Y, Li M, Cheng J, Jiang H. Ge J, et al. J Dent Res. 2015 Jul;94(7):936-44. doi: 10.1177/0022034515578908. Epub 2015 Mar 27. J Dent Res. 2015. PMID: 25818585 - MicroRNA expression signature for Satb2-induced osteogenic differentiation in bone marrow stromal cells.
Gong Y, Xu F, Zhang L, Qian Y, Chen J, Huang H, Yu Y. Gong Y, et al. Mol Cell Biochem. 2014 Feb;387(1-2):227-39. doi: 10.1007/s11010-013-1888-z. Epub 2013 Nov 12. Mol Cell Biochem. 2014. PMID: 24218084 - SATB1 and 2 in colorectal cancer.
Brocato J, Costa M. Brocato J, et al. Carcinogenesis. 2015 Feb;36(2):186-91. doi: 10.1093/carcin/bgu322. Epub 2014 Dec 27. Carcinogenesis. 2015. PMID: 25543122 Free PMC article. Review. - The role of SATB2 in skeletogenesis and human disease.
Zhao X, Qu Z, Tickner J, Xu J, Dai K, Zhang X. Zhao X, et al. Cytokine Growth Factor Rev. 2014 Feb;25(1):35-44. doi: 10.1016/j.cytogfr.2013.12.010. Epub 2013 Dec 25. Cytokine Growth Factor Rev. 2014. PMID: 24411565 Review.
Cited by
- Molecular Subtypes and Prognostic Models for Predicting Prognosis of Lung Adenocarcinoma based on MiRNA-related Genes.
Wei Y, Zhong W, Bi Y, Liu X, Zhou Q, Liu J, Wang M, Zhang H, Chen M. Wei Y, et al. Curr Med Chem. 2024;31(34):5620-5637. doi: 10.2174/0929867331666230914151943. Curr Med Chem. 2024. PMID: 37711127 - Oncogenic and tumor suppressor function of MEIS and associated factors.
Gİrgİn B, KaradaĞ-Alpaslan M, KocabaŞ F. Gİrgİn B, et al. Turk J Biol. 2020 Dec 14;44(6):328-355. doi: 10.3906/biy-2006-25. eCollection 2020. Turk J Biol. 2020. PMID: 33402862 Free PMC article. - Anti-cancer targets and molecular mechanisms of formononetin in treating osteosarcoma based on network pharmacology.
Chen L, Zhou Y, Weng Z, Liu S, Li T, Wang Y, Yang Y, Liu H, Huang W. Chen L, et al. Aging (Albany NY). 2023 Oct 20;15(20):11489-11507. doi: 10.18632/aging.205139. Epub 2023 Oct 20. Aging (Albany NY). 2023. PMID: 37870753 Free PMC article. - Satb2 regulates proliferation and nuclear integrity of pre-osteoblasts.
Dowrey T, Schwager EE, Duong J, Merkuri F, Zarate YA, Fish JL. Dowrey T, et al. Bone. 2019 Oct;127:488-498. doi: 10.1016/j.bone.2019.07.017. Epub 2019 Jul 17. Bone. 2019. PMID: 31325654 Free PMC article. - Comprehensive clinicopathologic, molecular, and immunologic characterization of colorectal carcinomas with loss of three intestinal markers, CDX2, SATB2, and KRT20.
Lee JA, Seo MK, Yoo SY, Cho NY, Kwak Y, Lee K, Kim JH, Kang GH. Lee JA, et al. Virchows Arch. 2022 Mar;480(3):543-555. doi: 10.1007/s00428-021-03260-w. Epub 2022 Jan 14. Virchows Arch. 2022. PMID: 35029777
References
- Szemes M., et al. (2006) Isolation and characterization of SATB2, a novel AT-rich DNA binding protein expressed in development- and cell-specific manner in the rat brain. Neurochem. Res., 31, 237–246. - PubMed
- FitzPatrick D.R., et al. (2003) Identification of SATB2 as the cleft palate gene on 2q32-q33. Hum. Mol. Genet., 12, 2491–2501. - PubMed
- Zhao X., et al. (2014) The role of SATB2 in skeletogenesis and human disease. Cytokine Growth Factor Rev., 25, 35–44. - PubMed
- Britanova O., et al. (2005) Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. Eur. J. Neurosci., 21, 658–668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials