Metabolic Pathways and Potencies of New Fentanyl Analogs - PubMed (original) (raw)
Review
Metabolic Pathways and Potencies of New Fentanyl Analogs
Maurice Wilde et al. Front Pharmacol. 2019.
Abstract
Up to now, little is known about the metabolic pathways of new fentanyl analogs that have recently emerged on the drug markets worldwide with high potential for producing addiction and severe adverse effects including coma and death. For some of the compounds, limited information on the metabolism has been published, however, for others so far no information is available. Considering the well characterized metabolism of the pharmaceutically used opioid fentanyl and the so far available data, the metabolism of the new fentanyl analogs can be anticipated to generally involve reactions like hydrolysis, hydroxylation (and further oxidation steps), _N_- and _O_-dealkylation and _O_-methylation. Furthermore, phase II metabolic reactions can be expected comprising glucuronide or sulfate conjugate formation. When analyzing blood and urine samples of acute intoxication cases or fatalities, the presence of metabolites can be crucial for confirmation of the uptake of such compounds and further interpretation. Here we present a review on the metabolic profiles of new fentanyl analogs responsible for a growing number of severe and fatal intoxications in the United States, Europe, Canada, Australia, and Japan in the last years, as assessed by a systematic search of the scientific literature and official reports.
Keywords: fentanyl analogs; fentanyl biotransformations; in vivo and in vitro metabolism; metabolic profile; novel synthetic opioids; receptor binding affinity; toxicity.
Figures
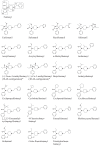
Figure 1
Chemical structures of fentanyl and reviewed fentanyl analogs with data on metabolism and/or potency available in the scientific literature. The structures marked with ‘∗’ show only one of the two enantiomers.
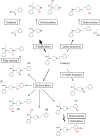
Figure 2
Schematic metabolic profile of fentanyl in humans, depicting the main biotransformations described in the literature. Main metabolic pathways are marked by bold arrows.
Similar articles
- In Vitro and In Vivo Metabolite Identification Studies for the New Synthetic Opioids Acetylfentanyl, Acrylfentanyl, Furanylfentanyl, and 4-Fluoro-Isobutyrylfentanyl.
Watanabe S, Vikingsson S, Roman M, Green H, Kronstrand R, Wohlfarth A. Watanabe S, et al. AAPS J. 2017 Jul;19(4):1102-1122. doi: 10.1208/s12248-017-0070-z. Epub 2017 Apr 5. AAPS J. 2017. PMID: 28382544 - A 2017-2019 Update on Acute Intoxications and Fatalities from Illicit Fentanyl and Analogs.
Brunetti P, Pirani F, Carlier J, Giorgetti R, Busardò FP, Lo Faro AF. Brunetti P, et al. J Anal Toxicol. 2021 Jul 10;45(6):537-554. doi: 10.1093/jat/bkaa115. J Anal Toxicol. 2021. PMID: 32860688 Review. - Acute Intoxications and Fatalities From Illicit Fentanyl and Analogues: An Update.
Pichini S, Solimini R, Berretta P, Pacifici R, Busardò FP. Pichini S, et al. Ther Drug Monit. 2018 Feb;40(1):38-51. doi: 10.1097/FTD.0000000000000465. Ther Drug Monit. 2018. PMID: 29120973 Review. - Toxicokinetic Studies and Analytical Toxicology of the New Synthetic Opioids Cyclopentanoyl-Fentanyl and Tetrahydrofuranoyl-Fentanyl.
Gampfer TM, Wagmann L, Richter MJ, Fischmann S, Westphal F, Meyer MR. Gampfer TM, et al. J Anal Toxicol. 2020 Apr 2;44(5):449-460. doi: 10.1093/jat/bkaa010. J Anal Toxicol. 2020. PMID: 32020187
Cited by
- Novel Vaccine That Blunts Fentanyl Effects and Sequesters Ultrapotent Fentanyl Analogues.
Barrientos RC, Bow EW, Whalen C, Torres OB, Sulima A, Beck Z, Jacobson AE, Rice KC, Matyas GR. Barrientos RC, et al. Mol Pharm. 2020 Sep 8;17(9):3447-3460. doi: 10.1021/acs.molpharmaceut.0c00497. Epub 2020 Aug 13. Mol Pharm. 2020. PMID: 32787282 Free PMC article. - Comparisons of fentanyl and sufentanil on recovery time after inguinal hernia repair in children: a randomized clinical trial.
Chen W, Guoyang H, Yu H, Xu Y. Chen W, et al. BMC Surg. 2024 Feb 14;24(1):55. doi: 10.1186/s12893-024-02346-x. BMC Surg. 2024. PMID: 38355485 Free PMC article. Clinical Trial. - Considerations for Cannabinoids in Perioperative Care by Anesthesiologists.
Laudanski K, Wain J. Laudanski K, et al. J Clin Med. 2022 Jan 22;11(3):558. doi: 10.3390/jcm11030558. J Clin Med. 2022. PMID: 35160010 Free PMC article. Review. - Deep learning-assisted sensitive detection of fentanyl using a bubbling-microchip.
Chen H, Kim S, Hardie JM, Thirumalaraju P, Gharpure S, Rostamian S, Udayakumar S, Lei Q, Cho G, Kanakasabapathy MK, Shafiee H. Chen H, et al. Lab Chip. 2022 Nov 22;22(23):4531-4540. doi: 10.1039/d2lc00478j. Lab Chip. 2022. PMID: 36331061 Free PMC article. - Molecular Mechanisms of Action of Novel Psychoactive Substances (NPS). A New Threat for Young Drug Users with Forensic-Toxicological Implications.
Giorgetti A, Pascali JP, Fais P, Pelletti G, Gabbin A, Franchetti G, Cecchetto G, Viel G. Giorgetti A, et al. Life (Basel). 2021 May 14;11(5):440. doi: 10.3390/life11050440. Life (Basel). 2021. PMID: 34068903 Free PMC article. Review.
References
- Andreasen M. F., Hardlei T. F., Rosendal I., Thomsen A. H., Johannsen M., Saedder E. (2017). “A fatal poisoning involving 2-fluorofentanyl,” in Proceedings of the 55th SOFT-TIAFT 2017 Annual Meeting, Boca Raton, FL.
- Åstrand A., Töreskog A., Watanabe S., Kronstrand R., Gréen H., Vikingsson S. (2018). Correlations between metabolism and structural elements of the alicyclic fentanyl analogs cyclopropyl fentanyl, cyclobutyl fentanyl, cyclopentyl fentanyl, cyclohexyl fentanyl and 2,2,3,3-tetramethylcyclopropyl fentanyl studied by human hepatocytes and LC-QTOF-MS. Arch. Toxicol. 93 95–106. 10.1007/s00204-018-2330-9 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources