The Roles of YAP/TAZ and the Hippo Pathway in Healthy and Diseased Skin - PubMed (original) (raw)
Review
The Roles of YAP/TAZ and the Hippo Pathway in Healthy and Diseased Skin
Emanuel Rognoni et al. Cells. 2019.
Abstract
Skin is the largest organ of the human body. Its architecture and physiological functions depend on diverse populations of epidermal cells and dermal fibroblasts. Reciprocal communication between the epidermis and dermis plays a key role in skin development, homeostasis and repair. While several stem cell populations have been identified in the epidermis with distinct locations and functions, there is additional heterogeneity within the mesenchymal cells of the dermis. Here, we discuss the current knowledge of how the Hippo pathway and its downstream effectors Yes-associated protein (YAP) and transcriptional coactivator with PDZ-binding motif (TAZ) contribute to the maintenance, activation and coordination of the epidermal and dermal cell populations during development, homeostasis, wound healing and cancer.
Keywords: Hippo signalling; fibroblasts; fibrosis; skin cancer; skin development; stem cells; wound healing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Figure 1
Morphology of the skin. The epidermis and dermis are separated by a BM. In the epidermis, multiple spatially distinct stem cell populations have been identified in the hair follicle bulge, isthmus and sebaceous gland, and their characteristic markers are shown in the colour coded legend. In the dermis, papillary fibroblasts are located in proximity to the BM and are embedded in thin collagen fibres. Reticular fibroblasts populate the central dermis and are surrounded by thick collagen bundles (grey). Preadipocytes are close to the DWAT where the mature adipocytes reside. In addition, specialised fibroblast subpopulations associate with the HF give rise to the DP, DS and APM. Endothelial cells form the blood vessels which are surrounded by pericytes. Sensory neurons are associated with the HF upper bulge SC population and different immune cell types populate different regions of the skin. Abbreviations: APM, arrector pili muscle; BM, basement membrane; Bu, bulge; DP, dermal papilla; DS, dermal sheath; DWAT, dermal white adipose tissue; SG, sebaceous gland.
Figure 2
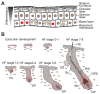
Yes-associated protein (YAP)/transcriptional coactivator with PDZ-binding motif (TAZ) activity in the IFE and HF development. (A) The IFE is a stratified squamous epithelium. It is divided into four main layers that are distinguished morphologically according to the differentiation status of the keratinocytes as they cease to proliferate and move upward to produce the skin’s barrier. Note that in adult IFE nuclear YAP/TAZ are restricted in cell clusters of the basal layer. (B) Early skin and HF development. During early skin development, cells of the epidermis and dermis are highly proliferative and highly positive for nuclear YAP/TAZ. Once the epidermis starts to stratify, only the proliferative cells in the basal layer maintain nuclear YAP/TAZ. HF development is initiated by an epidermal–mesenchymal cross-talk inducing condensation of mesenchymal cells beneath the BM which leads to the formation of a HF placode (HF stages 0–1). The HF placodes further matures into a hair germ (HF stages 1–2), which start to engulf the dermal papilla fibroblasts (HF stages 3–4). At HF stages 5–6 the dermal papilla is fully encapsulated; the HF epithelial cells differentiate into the distinct HF layers and the bulge and SG start to form. All cell compartments are clearly visible at HF stage 7–8 and the HF shaft emerges through the epidermis. YAP is nuclear in the placode and hair germ cells of the epidermis and dermis (HF stage 0–2) and then becomes more restricted to the highly proliferative basal epithelial cells (HF stage 3–8). YAP/TAZ are highly nuclear in the HF matrix, and there are additional cell cluster with nuclear YAP in the IFE and SG. Note that due to its wide-spread expression in skin, only nuclear YAP/TAZ are shown to indicate sites of YAPTAZ activity. Abbreviations: BM, basement membrane; Bu, bulge; DP, dermal papilla; HF, hair follicle; HG, hair germ; HS, hair shaft; IFE, interfollicular epidermis; IRS, inner root sheath; Mx matrix; ORS outer root sheath; SG, sebaceous gland.
Figure 3
YAP localisation during HF cycling. (A,B) Schematic of a HF in the resting phase (telogen) (A) and growth phase (anagen) (B). In telogen phase, YAP is mainly localised to the cytoplasm in bulge and DP cells and there are only scattered cluster of cells with nuclear YAP in the IFE and SG. During anagen the highly proliferative cells of the ORS and HF matrix display strong nuclear YAP localisation, while YAP is cytoplasmatic in differentiating cells of the IRS and HS. Beside cell clusters with nuclear YAP in the IFE and SG during anagen, some DP cells also display YAP in the nucleus. Note that only nuclear YAP is shown. (C) Immunostaining for YAP (red) and Itga6 (green) of mouse skin during telogen, early anagen and full anagen. Nuclei are stained with DAPI (blue). Note, the strong increase in nuclear YAP in the HG and infundibulum during anagen induction. Scale bars, 50 µm. Abbreviations: Bu, bulge; DP, dermal papilla; HF, hair follicle; HG, hair germ; HS, hair shaft; IFE, interfollicular epidermis; IRS, inner root sheath; Mx matrix; ORS outer root sheath; SG, sebaceous gland.
Figure 4
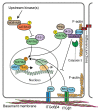
Regulation of YAP/TAZ in epidermal cells. Hippo signalling via MOB1A/MOB1B and LATS1/LATS2 inhibits YAP/TAZ via serine phosphorylation (yellow) to promote cytoplasmic retention. The kinases activating MOB1A/MOB1B and LATS1/LATS2 are not known. Integrin (ITG)–SRC signalling promotes YAP/TAZ nuclear localisation and TEAD binding. SRC can directly phosphorylate YAP/TAZ on tyrosine residues (orange) but may also act indirectly to activate Hippo signalling. A contractile F-actin-myosin cytoskeleton helps stabilise ITGβ1 adhesions and thus may contribute to SRC activation, while ITGβ4 adhesions are part of hemidesmosomal complexes that are stabilised by keratin intermediate filaments (not shown). At adherens junctions, α-catenin controls YAP/TAZ activity and phosphorylation by modulating its interaction with 14-3-3 and the PP2A phosphatase. In proliferating cells of the sebaceous gland, activation of caspase-3 cleaves α-catenin, thus facilitating the activation and nuclear translocation of YAP/TAZ. α-catenin can also inhibit ITGβ4-mediated direct activation of SRC. Putative nuclear interactions of YAP/TAZ with other transcription factors are also indicated.
Similar articles
- YAP and TAZ regulate skin wound healing.
Lee MJ, Byun MR, Furutani-Seiki M, Hong JH, Jung HS. Lee MJ, et al. J Invest Dermatol. 2014 Feb;134(2):518-525. doi: 10.1038/jid.2013.339. Epub 2013 Aug 9. J Invest Dermatol. 2014. PMID: 24108406 - Hippo-YAP/TAZ signalling in organ regeneration and regenerative medicine.
Moya IM, Halder G. Moya IM, et al. Nat Rev Mol Cell Biol. 2019 Apr;20(4):211-226. doi: 10.1038/s41580-018-0086-y. Nat Rev Mol Cell Biol. 2019. PMID: 30546055 Review. - The Hippo Signaling Pathway in Pancreatic Cancer.
Ansari D, Ohlsson H, Althini C, Bauden M, Zhou Q, Hu D, Andersson R. Ansari D, et al. Anticancer Res. 2019 Jul;39(7):3317-3321. doi: 10.21873/anticanres.13474. Anticancer Res. 2019. PMID: 31262852 Review. - Vitamin D3 analogue facilitates epithelial wound healing through promoting epithelial-mesenchymal transition via the Hippo pathway.
Wang D, Lin L, Lei K, Zeng J, Luo J, Yin Y, Li Y, Zhang L, Nie X, Zuo D, Sun L. Wang D, et al. J Dermatol Sci. 2020 Nov;100(2):120-128. doi: 10.1016/j.jdermsci.2020.08.015. Epub 2020 Sep 7. J Dermatol Sci. 2020. PMID: 32938565 - Hippo signaling modulation and its biological implications in urological malignancies.
Tong T, Huang M, Yan B, Lin B, Yu J, Teng Q, Li P, Pang J. Tong T, et al. Mol Aspects Med. 2024 Aug;98:101280. doi: 10.1016/j.mam.2024.101280. Epub 2024 Jun 12. Mol Aspects Med. 2024. PMID: 38870717 Review.
Cited by
- [Mechanism of cold atmospheric plasma in treatment of chronic skin ulcer].
Yuan N, Yang Y, Tan C, Ran X. Yuan N, et al. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Oct 15;38(10):1283-1288. doi: 10.7507/1002-1892.202404027. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 39433505 Free PMC article. Review. Chinese. - A feedback loop between plakophilin 4 and YAP signaling regulates keratinocyte differentiation.
Müller L, Gutschner T, Hatzfeld M. Müller L, et al. iScience. 2024 Aug 19;27(9):110762. doi: 10.1016/j.isci.2024.110762. eCollection 2024 Sep 20. iScience. 2024. PMID: 39286493 Free PMC article. - Skin Development and Disease: A Molecular Perspective.
Dermitzakis I, Chatzi D, Kyriakoudi SA, Evangelidis N, Vakirlis E, Meditskou S, Theotokis P, Manthou ME. Dermitzakis I, et al. Curr Issues Mol Biol. 2024 Jul 30;46(8):8239-8267. doi: 10.3390/cimb46080487. Curr Issues Mol Biol. 2024. PMID: 39194704 Free PMC article. Review. - Astragalus polysaccharide-containing 3D-printed scaffold for traumatized skin repair and proteomic study.
Du W, Wang Z, Han M, Zheng Y, Tao B, Pan N, Bao G, Zhuang W, Quan R. Du W, et al. J Cell Mol Med. 2024 Aug;28(16):e70023. doi: 10.1111/jcmm.70023. J Cell Mol Med. 2024. PMID: 39158533 Free PMC article. - A multiparametric analysis including single-cell and subcellular feature assessment reveals differential behavior of spheroid cultures on distinct ultra-low attachment plate types.
Vitacolonna M, Bruch R, Agaçi A, Nürnberg E, Cesetti T, Keller F, Padovani F, Sauer S, Schmoller KM, Reischl M, Hafner M, Rudolf R. Vitacolonna M, et al. Front Bioeng Biotechnol. 2024 Aug 2;12:1422235. doi: 10.3389/fbioe.2024.1422235. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39157442 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical