Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration - PubMed (original) (raw)
Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration
Jacopo Pizzicannella et al. Front Physiol. 2019.
Erratum in
- Erratum: Addendum: Engineered extracellular vesicles from human periodontal-ligament stem cells increase VEGF/VEGFR2 expression during bone regeneration.
Pizzicannella J, Gugliandolo A, Orsini T, Fontana A, Ventrella A, Mazzon E, Bramanti P, Diomede F, Trubiani O. Pizzicannella J, et al. Front Physiol. 2023 Mar 23;14:1148929. doi: 10.3389/fphys.2023.1148929. eCollection 2023. Front Physiol. 2023. PMID: 37035667 Free PMC article.
Abstract
Bone regeneration represents still a challenge, in particular for calvarium defects. Recently, the development of biomaterials with the addiction of stem cells is giving promising results for the treatment of bone defects. In particular, it was demonstrated that scaffolds enriched with mesenchymal stem cells (MSCs) and/or their derivatives, such as conditioned medium (CM) and extracellular vesicles (EVs), may improve bone regeneration. Moreover, given the deep link between osteogenesis and angiogenesis, a successful approach must also take into consideration the development of vascularization. In this work we evaluated the bone regeneration capacity of a collagen membrane (3D-COL) enriched with human periodontal-ligament stem cells (hPDLSCs) and CM or EVs or EVs engineered with polyethylenimine (PEI-EVs) in rats subjected to a calvarial defect. We evaluated also their capacity to induce angiogenic factors. At first, in vitro results showed an increased expression of osteogenic markers in hPDLSCs cultured with the 3D-COL and PEI-EVs, associated also with the increased protein levels of Vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR2). The increased expression of these proteins was confirmed also in vivo in rats implanted with the 3D-COL enriched with hPDLSCs and PEI-EVs. Moreover, histological examination evidenced in this group of rats the activation of bone regeneration and of the vascularization process. Also MicroCT imaging with morphometric analysis confirmed in rats transplanted with 3D-COL enriched with hPDLSCs and PEI-EVs an important regenerative process and a better integration level. All together, these results evidenced that the 3D-COL enriched with hPDLSCs and PEI-EVs may promote bone regeneration of calvaria defects, associated also with an increased vascularization.
Keywords: VEGF; VEGFR2; bone regeneration; collagen membrane; extracellular vesicles; mesenchymal stem cells; polyethylenimine.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Figure 1
hPDLSCs characterization. (A) Cytofluorimetric analysis of hPDLSCs culture. (B) Light microscopy representative picture of hPDLSCs adherent to the culture dish.
Figure 2
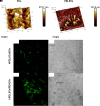
EVs and PEI-EV atomic force microscopic evaluation. (A1) EVs analyzed by tapping mode topographic 3D AFM technique showed a globular shape with a central depression. (A2) PEI-EVs analyzed by tapping mode topographic 3D AFM technique, showed a globular morphology with a less smooth surface. (B1) hPDLSCs incubated with WGA Alexa Fluor 488 stained EVs (green). (C1) hPDLSCs incubated with WGA Alexa Fluor 488 stained PEI-EVs (green). (B2,C2) Cell morphology observed at light transmission channel (gray scale). Mag, 63X.
Figure 3
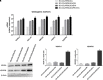
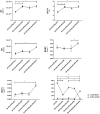
Gene expression of osteogenic markers and gene and protein expression for VEGF and VEGFR2 in vitro (n = 3). (A) Bar charts showed the gene expression for RUNX-2, COL1A1, BMP2/4, VEGF, and VEGFA in 3D-COL/hPDLSCs, 3D-COL/hPDLSCs/CM, 3D-COL/hPDLSCs/EVs, 3D-COL/hPDLSCs/PEI-EVs. Results showed the significant upregulation of these markers in 3D-COL/hPDLSCs/PEI-EVs compared to 3D-COL/hPDLSCs. (B) Protein specific bands and densitometric analysis of VEGFA and VEGFR2 in 3D-COL/hPDLSCs, 3D-COL/hPDLSCs/CM, 3D-COL/hPDLSCs/EVs, 3D-COL/hPDLSCs/PEI-EVs. Western blot analysis of VEGF and VEGFR2 in vitro evidenced increased levels for both proteins in 3D-COL/hPDLSCs/PEI-EVs compared to the other groups. ∗p < 0.05 3D-COL/hPDLSCs/PEI-EVs compared to 3D-COL/hPDLSCs.
Figure 4
In vivo VEGFA and VEGFR2 expression. Immunofluorescence staining of VEGFA and VEGFR2 showed the presence of the protein in semithin section samples obtained after 6 weeks of grafting in rat calvaria. Panels (A1,A2, B1,B2, C1,C2, D1,D2, E1,E2) showed the expression of VEGFA. Panels (A3,A4, B3,B4, C3,C4, D3,D4, E3,E4) showed the expression of VEGFR2. (A1,A3) 3D-COL at green fluorescent channel, (A2,A4) 3D-COL at light transmission channel. (B1,B3) 3D-COL/hPDLSCs at green fluorescent channel, (B2,B4) 3D-COL /hPDLSCs at light transmission channel. (C1,C3) 3D-COL/hPDLSCs/CM at green fluorescent channel, (C2,C4) 3D-COL/hPDLSCs/CM at light transmission channel. (D1,D3) 3D-COL/hPDLSCs/EVs at green fluorescent channel, (D2,D4) 3D-COL/hPDLSCs/EVs at light transmission channel. (E1,E3) 3D-COL/hPDLSCs/PEI-EVs at green fluorescent channel, (E2,E4) 3D-COL/hPDLSCs/PEI-EVs at light transmission channel. Mag, 20X; C, mouse calvaria; ∗3D-COL.
Figure 5
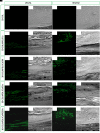
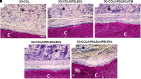
Histologic evaluation. Histologic view observed at light microscopy after 6 weeks of grafting and stained with acid fuchsin and methylene blue solution. (A) 3D-COL. (B) 3D-COL/hPDLSCs. (C) 3D-COL/hPDLSCs/CM. (D) 3D-COL/hPDLSCs/EVs. (E) 3D-COL/hPDLSCs/PEI-EVs. Mag, 40X; C, mouse calvaria; ∗3D-COL.
Figure 6
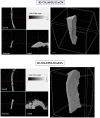
Micro-computed tomography (CT) analyses. 3D virtual histologic evaluation in volume rendering visualization modality of 3D-COL and 3D-COL/hPDLSCs, with 2D virtual sectioning in the 3 orthogonal planes.
Figure 7
Micro-computed tomography (CT) analyses. 3D virtual histologic evaluation in volume rendering visualization modality of 3D-COL/hPDLSCs/CM and 3D-COL/hPDLSCs/EVs, with 2D virtual sectioning in the 3 orthogonal planes.
Figure 8
Micro-computed tomography (CT) analyses. 3D virtual histologic evaluation in volume rendering visualization modality of 3D-COL/hPDLSCs/PEI-EVs, with 2D virtual sectioning in the 3 orthogonal planes.
Figure 9
Morphometric analysis. BV, BV/TV, BS (2D), BS/BV, BS/TV, connectivity and Euler number evidenced a better regenerative process in 3D-COL/hPDLSCs/PEI-EVs group. ∗P < 0.05, ∗∗p < 0.01.
Similar articles
- A novel role in skeletal segment regeneration of extracellular vesicles released from periodontal-ligament stem cells.
Diomede F, D'Aurora M, Gugliandolo A, Merciaro I, Ettorre V, Bramanti A, Piattelli A, Gatta V, Mazzon E, Fontana A, Trubiani O. Diomede F, et al. Int J Nanomedicine. 2018 Jun 29;13:3805-3825. doi: 10.2147/IJN.S162836. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29988728 Free PMC article. - 3D Printing PLA/Gingival Stem Cells/ EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment.
Pizzicannella J, Diomede F, Gugliandolo A, Chiricosta L, Bramanti P, Merciaro I, Orsini T, Mazzon E, Trubiani O. Pizzicannella J, et al. Int J Mol Sci. 2019 Jul 2;20(13):3256. doi: 10.3390/ijms20133256. Int J Mol Sci. 2019. PMID: 31269731 Free PMC article. - Three-dimensional printed PLA scaffold and human gingival stem cell-derived extracellular vesicles: a new tool for bone defect repair.
Diomede F, Gugliandolo A, Cardelli P, Merciaro I, Ettorre V, Traini T, Bedini R, Scionti D, Bramanti A, Nanci A, Caputi S, Fontana A, Mazzon E, Trubiani O. Diomede F, et al. Stem Cell Res Ther. 2018 Apr 13;9(1):104. doi: 10.1186/s13287-018-0850-0. Stem Cell Res Ther. 2018. PMID: 29653587 Free PMC article. - Human Oral Stem Cells, Biomaterials and Extracellular Vesicles: A Promising Tool in Bone Tissue Repair.
Trubiani O, Marconi GD, Pierdomenico SD, Piattelli A, Diomede F, Pizzicannella J. Trubiani O, et al. Int J Mol Sci. 2019 Oct 9;20(20):4987. doi: 10.3390/ijms20204987. Int J Mol Sci. 2019. PMID: 31600975 Free PMC article. Review. - Mesenchymal Stem Cell-Derived Extracellular Vesicles for Bone Defect Repair.
Wang D, Cao H, Hua W, Gao L, Yuan Y, Zhou X, Zeng Z. Wang D, et al. Membranes (Basel). 2022 Jul 19;12(7):716. doi: 10.3390/membranes12070716. Membranes (Basel). 2022. PMID: 35877919 Free PMC article. Review.
Cited by
- Mesenchymal stromal cell-derived extracellular vesicles for bone regeneration therapy.
Murali VP, Holmes CA. Murali VP, et al. Bone Rep. 2021 May 17;14:101093. doi: 10.1016/j.bonr.2021.101093. eCollection 2021 Jun. Bone Rep. 2021. PMID: 34095360 Free PMC article. Review. - Analogies and Differences Between Dental Stem Cells: Focus on Secretome in Combination with Scaffolds in Neurological Disorders.
Santilli F, Fabrizi J, Santacroce C, Caissutti D, Spinello Z, Candelise N, Lancia L, Pulcini F, Delle Monache S, Mattei V. Santilli F, et al. Stem Cell Rev Rep. 2024 Jan;20(1):159-174. doi: 10.1007/s12015-023-10652-9. Epub 2023 Nov 14. Stem Cell Rev Rep. 2024. PMID: 37962698 Free PMC article. Review. - The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases.
Wan X, Zhang W, Dai L, Chen L. Wan X, et al. Curr Issues Mol Biol. 2024 Aug 23;46(9):9269-9285. doi: 10.3390/cimb46090548. Curr Issues Mol Biol. 2024. PMID: 39329900 Free PMC article. Review. - Designer Exosomes: A New Platform for Biotechnology Therapeutics.
Jafari D, Shajari S, Jafari R, Mardi N, Gomari H, Ganji F, Forouzandeh Moghadam M, Samadikuchaksaraei A. Jafari D, et al. BioDrugs. 2020 Oct;34(5):567-586. doi: 10.1007/s40259-020-00434-x. BioDrugs. 2020. PMID: 32754790 Free PMC article. Review. - Extracellular vesicles: From bone development to regenerative orthopedics.
Davies OG. Davies OG. Mol Ther. 2023 May 3;31(5):1251-1274. doi: 10.1016/j.ymthe.2023.02.021. Epub 2023 Mar 3. Mol Ther. 2023. PMID: 36869588 Free PMC article. Review.
References
- Angelini G., Boncompagni S., De Maria P., De Nardi M., Fontana A., Gasbarri C., et al. (2007). Layer-by-layer deposition of shortened nanotubes or polyethylene glycol-derivatized nanotubes on liposomes: a tool for increasing liposome stability. Carbon 45 2479–2485. 10.1016/j.carbon.2007.08.038 - DOI
- Angelini G., Boncompagni S., De Maria P., Fontana A., Gasbarri C., Siani G. (2008). Kinetic evaluation of the effect of layer by layer deposition of polyelectrolytes on the stability of POPC liposomes. Colloids Surf. A Physicochem. Eng. Asp. 322 234–238. 10.1016/j.colsurfa.2008.03.015 - DOI
- Ballerini P., Diomede F., Petragnani N., Cicchitti S., Merciaro I., Cavalcanti M. F. X. B., et al. (2017). Conditioned medium from relapsing-remitting multiple sclerosis patients reduces the expression and release of inflammatory cytokines induced by LPS-gingivalis in THP-1 and MO3.13 cell lines. Cytokine 96 261–272. 10.1016/j.cyto.2017.04.022 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources