Greater Efficacy of Black Ginseng (CJ EnerG) over Red Ginseng against Lethal Influenza A Virus Infection - PubMed (original) (raw)
Comparative Study
. 2019 Aug 13;11(8):1879.
doi: 10.3390/nu11081879.
Son-Woo Kim 2, Su-Jin Park 1, Semi Kim 1, Kwang-Min Yu 1, Seong Gyu Kim 3, Seung Hun Lee 1, Yong-Ki Seo 2, Nam-Hoon Cho 2, Kimoon Kang 2, Do Y Soung 4, Young-Ki Choi 5 6
Affiliations
- PMID: 31412594
- PMCID: PMC6723933
- DOI: 10.3390/nu11081879
Comparative Study
Greater Efficacy of Black Ginseng (CJ EnerG) over Red Ginseng against Lethal Influenza A Virus Infection
Eun-Ha Kim et al. Nutrients. 2019.
Abstract
Black ginseng (BG, CJ EnerG), prepared via nine repeated cycles of steaming and drying of fresh ginseng, contains more accessible acid polysaccharides and smaller and less polar ginsenosides than red ginseng (RG) processed only once. Because RG exhibits the ability to increase host protection against viral respiratory infections, we investigated the antiviral effects of BG. Mice were orally administered either BG or RG extract at 10 mg/kg bw daily for two weeks. Mice were then infected with a A(H1N1) pdm09 (A/California/04/2009) virus and fed extracts for an additional week. Untreated, infected mice were assigned to either the negative control, without treatments, or the positive control, treated with Tamiflu. Infected mice were monitored for 14 days to determine the survival rate. Lung tissues were evaluated for virus titer and by histological analyses. Cytokine levels were measured in bronchoalveolar lavage fluid. Mice treated with BG displayed a 100% survival rate against infection, while mice treated with RG had a 50% survival rate. Further, mice treated with BG had fewer accumulated inflammatory cells in bronchioles following viral infection than did mice treated with RG. BG also enhanced the levels of GM-CSF and IL-10 during the early and late stages of infection, respectively, compared to RG. Thus, BG may be useful as an alternative antiviral adjuvant to modulate immune responses to influenza A virus.
Keywords: antiviral; black ginseng; cytokines; influenza A virus; oral administration.
Conflict of interest statement
E.-H.K., S.-J.P., S.K., K.-M.Y., S.G.K., S.H.L., and Y.-K.C. declare the absence of any conflicts of interest. S.-W.K., Y.-K.S., N.-H.C., K.K., and D.Y.S. are employees of the CJ CheilJedang Corporation. However, the founding sponsors had no role in the performance of the experiments; in the collection, analyses, interpretation, validation, or visualization of data; and in the writing of the original draft that are associated with the animal study.
Figures
Figure 1
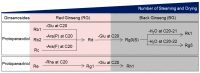
Transformation of the ginsenoside profile of ginseng with increased numbers of steaming and drying cycles. There are two types of ginsenosides: protopanaxadiol-type saponins (e.g., Rb1, Rb2, Rc, Rd, Rg3, Rg5, and Rk1) with sugar moieties attached to hydroxyl groups at C3 and C20 and protopanaxatriol type saponins (e.g., Re, Rg1, and Rh1) with sugar moieties attached to hydroxyl groups at C3, C6, and C20. The outer residues from position C20 of Rb1, Rb2 and Rc are glucose, arabinose (pyranose form), and arabinose (furanose form), respectively. These outer residues are removed to achieve Rd. The remaining glucose of Rd at C20 can be deleted to form Rg3. Sequentially, Rk1 with the double bond at C20-21 and Rg5 with a double bond at C20-22 are derived from Rg3 by dehydration at C20. Re, a protopanaxatriol type, can also be transformed to Rg1 after deletion of the rhamnose residue at C6. The outer glucose residue of Rg1 at C20 is removed to form Rh1. Glu: glucose; Ara(P): arabinose (pyranose form); Ara(F): arabinose (furanose form); Rha: rhamnose.
Figure 2
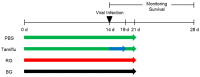
Schematic diagram of animal experiments. Mice were orally administered either red ginseng (RG) or black ginseng (BG) (10 mg/kg bw daily) for 14 days. After challenge with A/California/04/2009 virus, mice continuously received either RG or BG for an additional week. As a negative control, mice that received phosphate buffered saline (PBS) daily for 14 days were also infected with virus. The positive control group was treated with Tamiflu daily for 5 days post-infection and then with PBS for 2 additional days. All mice were monitored for 14 days post-infection to measure survival.
Figure 3
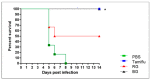
Treatment with BG results in a higher survival rate in influenza virus-infected mice than does treatment with RG. Survival was monitored for 14 days after influenza A virus infection in PBS-, Tamiflu-, RG-, and BG-treated mice (n = 6 per group). * p < 0.05 vs. PBS.
Figure 4
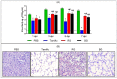
Treatment with BG improves antiviral activity and prevents histopathological alterations following viral infection. (A) PBS-, Tamiflu-, RG-, and BG- treated mice were euthanized to collect lung tissues at 1, 3, 5, and 7 dpi. Uninfected mouse lungs were also isolated to use as an intact control (dotted line). Lung viral titer was determined in homogenized tissues by the hemagglutination test. Values are mean (n = 6 per group at each time point) ± SEM. *, p < 0.05 vs. PBS; #, p < 0.05 vs. Tamiflu. (B) At 5 dpi, paraffin-embedded lung samples were prepared from infected mice treated with either PBS, Tamiflu, RG, or BG. Representative histological sections of lung tissues stained with H&E to visualize inflammatory lesions (magnification: × 100).
Figure 5
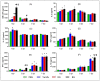
BG treatment further enhances production of cytokines in BALF. At 1, 3, 5, and 7 dpi BALF samples were harvested from PBS-, Tamiflu-, RG-, and BG- treated mouse lungs. Bronchoalveolar lavage fluid (BALF) samples were also isolated from uninfected mice for use as an intact control (dotted line). Cytokine production was analyzed in lung BALF by BioPlex analysis. (A) Granulocyte-macrophage colony-stimulating factor (GM-CSF), (B) Interleukin 2 (IL-2), (C) IL-1β, (D) tumor necrosis factor-alpha (TNF-α), (E) interferon-gamma (IFN-γ), and (F) IL-10. Values are the mean (n = 6 per group at each time point) ± SEM. *, p < 0.05 vs. PBS; #, p < 0.05 vs. Tamiflu; $, p < 0.05 vs. RG.
Figure 6
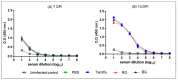
BG treatment does not disturb the normal development of IgG following the first virus inoculation. PBS-, Tamiflu-, RG-, and BG- treated mice were euthanized at 7 and 14 dpi to collect sera. Serum was also isolated from uninfected mice for use as an intact control. Anti-influenza A virus IgG titers were measured in sera by ELISA. Data are representative of three independent experiments. Values are the mean (n = 6 per group at each time point) ± SEM.
Figure 7
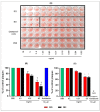
BG exhibits antiviral activities against A/California/04/2009 in vitro. (A) Hemagglutination inhibition (HI) assay conducted with PBS, RG, BG, and Oseltamivir with A/California/04/2009 in 96-well plates. A total of 0.02 to 10 mg/mL of each extract incubated with 4 to 8 HA unit of A/California/04/2009 virus for 60 min at RT and HI assay was conducted with 0.7% of turkey red blood cells (RBCs). Viral neutralization (plaque formation) assessments were performed in Madin-Darby Canine Kidney (MDCK) cells with (B) the pretreatment and (C) the posttreatment of BG against viral replication. The antiviral effect of BG was compared with RG and Oseltamivir treatment. Results are presented as the percentage of plaque reduction in each treatment group relative to the plaque formation in the PBS treatment group (negative control). Values are the mean ± SD *, p < 0.05 vs. PBS; †, p < 0.01 vs. PBS; ‡, p < 0.001 vs. PBS. N.D: Not detected.
Similar articles
- Protective effect of Korean red ginseng extract on the infections by H1N1 and H3N2 influenza viruses in mice.
Yoo DG, Kim MC, Park MK, Song JM, Quan FS, Park KM, Cho YK, Kang SM. Yoo DG, et al. J Med Food. 2012 Oct;15(10):855-62. doi: 10.1089/jmf.2012.0017. Epub 2012 Aug 2. J Med Food. 2012. PMID: 22856395 Free PMC article. - Antiviral Activity of Fermented Ginseng Extracts against a Broad Range of Influenza Viruses.
Wang Y, Jung YJ, Kim KH, Kwon Y, Kim YJ, Zhang Z, Kang HS, Wang BZ, Quan FS, Kang SM. Wang Y, et al. Viruses. 2018 Sep 1;10(9):471. doi: 10.3390/v10090471. Viruses. 2018. PMID: 30200514 Free PMC article. - San Wu Huangqin Decoction, a Chinese Herbal Formula, Inhibits Influenza a/PR/8/34 (H1N1) Virus Infection In Vitro and In Vivo.
Ma Q, Yu Q, Xing X, Liu S, Shi C, Luo J. Ma Q, et al. Viruses. 2018 Mar 9;10(3):117. doi: 10.3390/v10030117. Viruses. 2018. PMID: 29522425 Free PMC article. - Ginseng, the natural effectual antiviral: Protective effects of Korean Red Ginseng against viral infection.
Im K, Kim J, Min H. Im K, et al. J Ginseng Res. 2016 Oct;40(4):309-314. doi: 10.1016/j.jgr.2015.09.002. Epub 2015 Sep 16. J Ginseng Res. 2016. PMID: 27746682 Free PMC article. Review. - Pharmacological Efficacy of Ginseng against Respiratory Tract Infections.
Alsayari A, Muhsinah AB, Almaghaslah D, Annadurai S, Wahab S. Alsayari A, et al. Molecules. 2021 Jul 5;26(13):4095. doi: 10.3390/molecules26134095. Molecules. 2021. PMID: 34279434 Free PMC article. Review.
Cited by
- Historical evolution and processing mechanism of 'nine steaming and nine drying' of traditional Chinese medicine preparation.
Li YK, Chen Z, Zhang C. Li YK, et al. Pharm Biol. 2024 Dec;62(1):436-446. doi: 10.1080/13880209.2024.2354345. Epub 2024 May 16. Pharm Biol. 2024. PMID: 38755954 Free PMC article. Review. - The Role of Traditional Chinese Medicine and Chinese Pharmacopoeia in the Evaluation and Treatment of COVID-19.
Gasmi A, Noor S, Dadar M, Semenova Y, Menzel A, Gasmi Benahmed A, Bjørklund G. Gasmi A, et al. Curr Pharm Des. 2024;30(14):1060-1074. doi: 10.2174/0113816128217263240220060252. Curr Pharm Des. 2024. PMID: 38523518 Review. - Red Ginseng Attenuates the Hepatic Cellular Senescence in Aged Mice.
Lee DY, Arndt J, O'Connell JF, Egan JM, Kim Y. Lee DY, et al. Biology (Basel). 2024 Jan 8;13(1):36. doi: 10.3390/biology13010036. Biology (Basel). 2024. PMID: 38248467 Free PMC article. - Evaluation of Steaming and Drying of Black Sesame Seeds for Nine Cycles Using Grey-Correlation Analysis Based on Variation-Coefficient Weight.
Zhang Y, Wang J, Tan H, Lu X, Wang D, Wei Q. Zhang Y, et al. Molecules. 2023 Jul 7;28(13):5266. doi: 10.3390/molecules28135266. Molecules. 2023. PMID: 37446935 Free PMC article. - Antiviral Potential of the Genus Panax: An updated review on their effects and underlying mechanism of action.
Zhang Y, Zhong X, Xi Z, Li Y, Xu H. Zhang Y, et al. J Ginseng Res. 2023 Mar;47(2):183-192. doi: 10.1016/j.jgr.2022.11.003. Epub 2022 Nov 17. J Ginseng Res. 2023. PMID: 36926608 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources