Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma - PubMed (original) (raw)
Review
Pharmacogenetics of the systemic treatment in advanced hepatocellular carcinoma
Elena De Mattia et al. World J Gastroenterol. 2019.
Abstract
Hepatocellular carcinoma (HCC) accounts for the majority of primary liver cancers. To date, most patients with HCC are diagnosed at an advanced tumor stage, excluding them from potentially curative therapies (i.e., resection, liver transplantation, percutaneous ablation). Treatments with palliative intent include chemoembolization and systemic therapy. Among systemic treatments, the small-molecule multikinase inhibitor sorafenib has been the only systemic treatment available for advanced HCC over 10 years. More recently, other small-molecule multikinase inhibitors (e.g., regorafenib, lenvatinib, cabozantinib) have been approved for HCC treatment. The promising immune checkpoint inhibitors (e.g., nivolumab, pembrolizumab) are still under investigation in Europe while in the US nivolumab has already been approved by FDA in sorafenib refractory or resistant patients. Other molecules, such as the selective CDK4/6inhibitors (e.g., palbociclib, ribociclib), are in earlier stages of clinical development, and the c-MET inhibitor tivantinib did not show positive results in a phase III study. However, even if the introduction of targeted agents has led to great advances in patient response and survival with an acceptable toxicity profile, a remarkable inter-individual heterogeneity in therapy outcome persists and constitutes a significant problem in disease management. Thus, the identification of biomarkers that predict which patients will benefit from a specific intervention could significantly affect decision-making and therapy planning. Germ-line variants have been suggested to play an important role in determining outcomes of HCC systemic therapy in terms of both toxicity and treatment efficacy. Particularly, a number of studies have focused on the role of genetic polymorphisms impacting the drug metabolic pathway and membrane translocation as well as the drug mechanism of action as predictive/prognostic markers of HCC treatment. The aim of this review is to summarize and critically discuss the pharmacogenetic literature evidences, with particular attention to sorafenib and regorafenib, which have been used longer than the others in HCC treatment.
Keywords: Cytochromes; Genetic markers; Hepatocellular carcinoma; Immune checkpoint inhibitors; Pharmacogenetics; Regorafenib; Sorafenib; UDP glucuronosyltransferase 1A.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflict of interest.
Figures
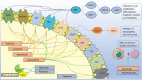
Figure 1
Mechanisms of action of the drugs covered in the text. Approved drugs are in the orange box while those under approval are in the grey box. Image created with Servier Medical Art (https://smart.servier.com/). FGFR1: Fibroblast growth factor receptor; PDGFR: Platelet-derived growth factor receptor; FLT3: Fms-related tyrosine kinase 3; VEGFR: Vascular endothelial growth factor receptor; TIE: Tyrosine kinase with immunoglobulin-like and EGF-like domains; HGFR: Hepatocyte growth factor receptor; PD1: Programmed cell death protein-1; PDL1/2: programmed cell death protein ligand 1/2; CDK: Cyclin-dependent kinases.
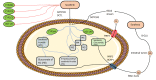
Figure 2
Schematic overview of sorafenib metabolism. Briefly, after oral administration, sorafenib enters hepatocytes by anion transporter family member (OATP1B, encoded by SLCO1B)-type carriers and cation transporter-1 (OCT1, encoded by SLC22A1). Within the hepatocytes, sorafenib undergoes phase I cytochrome P450 3A4 (CYP3A4)- and phase II UDP glucuronosyltransferase 1A9 (UGT1A9)-mediated metabolism to form M1-8 metabolites and sorafenib glucuronide (SG). After conjugation, SG is extensively secreted into the bile by a process that is mainly mediated by multidrug resistance protein (MRP) 2 (encoded by ABCC2) and breast cancer resistance protein BCRP (encoded by ABCG2) and into the bloodstream by MRP3 (encoded by ABCC3). A fraction of SG enters the intestinal lumen, where it could be a substrate for bacterial β-glucuronidases (B-GLU) that regenerate the parental drug sorafenib, which reenters the systemic circulation through the OATP1B3 carrier. CYP2B6, CYP2C8, CYP2C9, and UGT1A1 may interfere with sorafenib metabolism, being inhibited by sorafenib (see text for details). Image created with Servier Medical Art (https://smart.servier.com/).
Similar articles
- Molecular targeted and immune checkpoint therapy for advanced hepatocellular carcinoma.
Liu Z, Lin Y, Zhang J, Zhang Y, Li Y, Liu Z, Li Q, Luo M, Liang R, Ye J. Liu Z, et al. J Exp Clin Cancer Res. 2019 Nov 4;38(1):447. doi: 10.1186/s13046-019-1412-8. J Exp Clin Cancer Res. 2019. PMID: 31684985 Free PMC article. Review. - Molecular therapies for HCC: Looking outside the box.
Faivre S, Rimassa L, Finn RS. Faivre S, et al. J Hepatol. 2020 Feb;72(2):342-352. doi: 10.1016/j.jhep.2019.09.010. J Hepatol. 2020. PMID: 31954496 Review. - Comparative Efficacy of Cabozantinib and Regorafenib for Advanced Hepatocellular Carcinoma.
Kelley RK, Mollon P, Blanc JF, Daniele B, Yau T, Cheng AL, Valcheva V, Marteau F, Guerra I, Abou-Alfa GK. Kelley RK, et al. Adv Ther. 2020 Jun;37(6):2678-2695. doi: 10.1007/s12325-020-01378-y. Epub 2020 May 18. Adv Ther. 2020. PMID: 32424805 Free PMC article. - Cabozantinib in patients with hepatocellular carcinoma failing previous treatment with sorafenib.
Personeni N, Pressiani T, Rimassa L. Personeni N, et al. Future Oncol. 2019 Jul;15(21):2449-2462. doi: 10.2217/fon-2019-0026. Epub 2019 Jun 17. Future Oncol. 2019. PMID: 31204849 Review. - Efficacy of annexin A3 blockade in sensitizing hepatocellular carcinoma to sorafenib and regorafenib.
Tong M, Che N, Zhou L, Luk ST, Kau PW, Chai S, Ngan ES, Lo CM, Man K, Ding J, Lee TK, Ma S. Tong M, et al. J Hepatol. 2018 Oct;69(4):826-839. doi: 10.1016/j.jhep.2018.05.034. Epub 2018 Jun 7. J Hepatol. 2018. PMID: 29885413
Cited by
- miR-199a-3p increases the anti-tumor activity of palbociclib in liver cancer models.
Callegari E, Guerriero P, Bassi C, D'Abundo L, Frassoldati A, Simoni E, Astolfi L, Silini EM, Sabbioni S, Negrini M. Callegari E, et al. Mol Ther Nucleic Acids. 2022 Jul 20;29:538-549. doi: 10.1016/j.omtn.2022.07.015. eCollection 2022 Sep 13. Mol Ther Nucleic Acids. 2022. PMID: 36035756 Free PMC article. - Potential of novel colchicine dosage schedule for the palliative treatment of advanced hepatocellular carcinoma.
Lin ZY, Yeh ML, Huang CI, Chen SC, Huang CF, Huang JF, Dai CY, Yu ML, Chuang WL. Lin ZY, et al. Kaohsiung J Med Sci. 2021 Jul;37(7):616-623. doi: 10.1002/kjm2.12374. Epub 2021 Mar 3. Kaohsiung J Med Sci. 2021. PMID: 33655688 Free PMC article. Clinical Trial. - Molecular Bases of Drug Resistance in Hepatocellular Carcinoma.
Marin JJG, Macias RIR, Monte MJ, Romero MR, Asensio M, Sanchez-Martin A, Cives-Losada C, Temprano AG, Espinosa-Escudero R, Reviejo M, Bohorquez LH, Briz O. Marin JJG, et al. Cancers (Basel). 2020 Jun 23;12(6):1663. doi: 10.3390/cancers12061663. Cancers (Basel). 2020. PMID: 32585893 Free PMC article. Review. - LINC00244 suppresses cell growth and metastasis in hepatocellular carcinoma by downregulating programmed cell death ligand 1.
Sun Z, Xue C, Li J, Zhao H, Du Y, Du N. Sun Z, et al. Bioengineered. 2022 Mar;13(3):7635-7647. doi: 10.1080/21655979.2022.2050073. Bioengineered. 2022. PMID: 35266439 Free PMC article. - The anti-tumor efficacy of 20(S)-protopanaxadiol, an active metabolite of ginseng, according to fasting on hepatocellular carcinoma.
Li W, Wang Y, Zhou X, Pan X, Lü J, Sun H, Xie Z, Chen S, Gao X. Li W, et al. J Ginseng Res. 2022 Jan;46(1):167-174. doi: 10.1016/j.jgr.2021.06.002. Epub 2021 Jun 10. J Ginseng Res. 2022. PMID: 35058733 Free PMC article.
References
- Llovet JM, Zucman-Rossi J, Pikarsky E, Sangro B, Schwartz M, Sherman M, Gores G. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. - PubMed
- Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. - PubMed
- Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47:82–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous