Anticipated Pain During Intrauterine Device Insertion - PubMed (original) (raw)
Randomized Controlled Trial
Anticipated Pain During Intrauterine Device Insertion
Tegan A Hunter et al. J Pediatr Adolesc Gynecol. 2020 Feb.
Abstract
Study objective: To identify predictors of anticipated pain with intrauterine device (IUD) insertion in adolescents and young women.
Design: We performed linear regression to identify demographic, sexual/gynecologic history, and mood covariates associated with anticipated pain using a visual analogue scale pain score collected as part of a single-blind randomized trial of women who received a 13.5-mg levonorgestrel IUD.
Setting: Three academic family planning clinics in Philadelphia Pennsylvania.
Participants: Ninety-three adolescents and young adult women aged 14-22 years.
Intervention: Participants received either a 1% lidocaine or sham paracervical block.
Main outcome measures: Anticipated pain measured using a visual analogue scale before and perceived pain at 6 time points during the IUD insertion procedure.
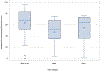
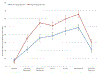
Results: Black or African American participants had a median anticipated pain score of 68 (interquartile range [IQR], 52-83), White participants had a median anticipated pain of 51 (IQR, 35-68), whereas participants of other races had a median anticipated pain score of 64 (IQR, 36-73); P = .012. In multivariate analysis, race was the only covariate that significantly predicted anticipated pain at IUD insertion. Women with anticipated pain scores above the median had significantly higher perceived pain during all timepoints of the IUD insertion procedure.
Conclusion: Increased anticipated pain is associated with increased perceived pain with IUD insertion. Black adolescent women experience greater anticipated pain with IUD insertion. This population might benefit from counseling and clinical measures to reduce this barrier to IUD use.
Keywords: Adolescent; Contraceptive devices; Intrauterine devices; Pain.
Copyright © 2020 North American Society for Pediatric and Adolescent Gynecology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Disclosure/Conflict of Interest:
TA Hunter has nothing to disclose. SR Sonalkar has nothing to disclose. CA Schreiber is a consultant for Exeltis Pharmaceuticals and received research funding from Bayer Pharmaceuticals, Medicines360, ContraMed, and NICHD. LK Perriera has nothing to disclose. MD Sammel has nothing to disclose. AY Akers is a consultant for the Merck HPV advisory board and is a member of the Mylan Inc Women’s Health Advisory Board.
Figures
Figure 1)
Anticipated pain of IUD insertion as measured by a 100 mm Visual Analog Scale, stratified by race category. The box represents the interquartile range, the horizontal line represents the median, and diamonds represent the mode. Whiskers represent the scores outside the interquartile range. Circles indicate outliers.
Figure 2)
Adjusted perceived pain VAS scores (±SE) in women with high and low anticipated pain. High anticipated pain scores were those above the median (red); low anticipated pain scores were those below the median (blue). VAS scores were adjusted for race, age, and randomization group. Difference in VAS scores with P<0.05 are marked with a *.
Similar articles
- Satisfaction With the Intrauterine Device Insertion Procedure Among Adolescent and Young Adult Women.
Akers AY, Harding J, Perriera LK, Schreiber C, Garcia-Espana JF, Sonalkar S. Akers AY, et al. Obstet Gynecol. 2018 Jun;131(6):1130-1136. doi: 10.1097/AOG.0000000000002596. Obstet Gynecol. 2018. PMID: 29742656 Free PMC article. Clinical Trial. - Reducing Pain During Intrauterine Device Insertion: A Randomized Controlled Trial in Adolescents and Young Women.
Akers AY, Steinway C, Sonalkar S, Perriera LK, Schreiber C, Harding J, Garcia-Espana JF. Akers AY, et al. Obstet Gynecol. 2017 Oct;130(4):795-802. doi: 10.1097/AOG.0000000000002242. Obstet Gynecol. 2017. PMID: 28885425 Clinical Trial. - Pain Perception during Levonorgestrel-releasing Intrauterine Device Insertion in Nulliparous Women: A Systematic Review.
Anthoulakis C, Iordanidou E, Vatopoulou A. Anthoulakis C, et al. J Pediatr Adolesc Gynecol. 2018 Dec;31(6):549-556.e4. doi: 10.1016/j.jpag.2018.05.008. Epub 2018 Jun 8. J Pediatr Adolesc Gynecol. 2018. PMID: 29890206 - Analgesia efficacy of lidocaine transfused by a novel disposable injectable cervical dilator during intrauterine device removal procedure: A randomized clinical trial.
Wang Y, Chen Q, Liu Z, Chen Y, Zheng Y, Guo J, Zhou F, Lv N, Zhao J, Shen S, Yuan Q, Tong J. Wang Y, et al. Contraception. 2024 Jul;135:110439. doi: 10.1016/j.contraception.2024.110439. Epub 2024 Mar 27. Contraception. 2024. PMID: 38552820 Clinical Trial. - Uterine or paracervical lidocaine application for pain control during intrauterine contraceptive device insertion: a meta-analysis of randomised controlled trials.
Perez-Lopez FR, Martinez-Dominguez SJ, Perez-Roncero GR, Hernandez AV. Perez-Lopez FR, et al. Eur J Contracept Reprod Health Care. 2018 Jun;23(3):207-217. doi: 10.1080/13625187.2018.1469124. Epub 2018 May 24. Eur J Contracept Reprod Health Care. 2018. PMID: 29792756
Cited by
- U.S. Selected Practice Recommendations for Contraceptive Use, 2024.
Curtis KM, Nguyen AT, Tepper NK, Zapata LB, Snyder EM, Hatfield-Timajchy K, Kortsmit K, Cohen MA, Whiteman MK; Contributors. Curtis KM, et al. MMWR Recomm Rep. 2024 Aug 8;73(3):1-77. doi: 10.15585/mmwr.rr7303a1. MMWR Recomm Rep. 2024. PMID: 39106301 Free PMC article. - Differing Approaches to Pain Management for Intrauterine Device Insertion and Maintenance: A Scoping Review.
Rahman M, King C, Saikaly R, Sosa M, Sibaja K, Tran B, Tran S, Morello P, Yeon Seo S, Yeon Seo Y, Jacobs RJ. Rahman M, et al. Cureus. 2024 Mar 8;16(3):e55785. doi: 10.7759/cureus.55785. eCollection 2024 Mar. Cureus. 2024. PMID: 38586685 Free PMC article. - Teaching About Contraception: Adolescent Attitudes Surrounding Sexual Education.
Zeglin A, Lazebnik R. Zeglin A, et al. Open Access J Contracept. 2023 Dec 1;14:181-188. doi: 10.2147/OAJC.S402443. eCollection 2023. Open Access J Contracept. 2023. PMID: 38059115 Free PMC article. Review. - Advancing Pain Management Protocols for Intrauterine Device Insertion: Integrating Evidence-Based Strategies Into Clinical Practice.
Estevez E, Hem-Lee-Forsyth S, Viechweg N, John S, Menor SP. Estevez E, et al. Cureus. 2024 Jun 25;16(6):e63125. doi: 10.7759/cureus.63125. eCollection 2024 Jun. Cureus. 2024. PMID: 39055461 Free PMC article. Review. - Analgesic effects of LI4 acupuncture during intrauterine device insertion: a randomized controlled clinical trial.
Erdoğan P, Yardımcı H. Erdoğan P, et al. Arch Gynecol Obstet. 2023 Oct;308(4):1361-1368. doi: 10.1007/s00404-023-07106-5. Epub 2023 Jul 19. Arch Gynecol Obstet. 2023. PMID: 37466690 Clinical Trial.
References
- Finer LB. Unintended pregnancy among U.S. adolescents: accounting for sexual activity. J Adolesc Health 2010;47(3):312–4. - PubMed
- Daniels K. Current Contraceptive Status Among Women Aged 15–49: United States, 2015–2017. 2018;(327):8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical