SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor - PubMed (original) (raw)
. 2020 Apr 16;181(2):271-280.e8.
doi: 10.1016/j.cell.2020.02.052. Epub 2020 Mar 5.
Hannah Kleine-Weber 2, Simon Schroeder 3, Nadine Krüger 4, Tanja Herrler 5, Sandra Erichsen 6, Tobias S Schiergens 7, Georg Herrler 8, Nai-Huei Wu 8, Andreas Nitsche 9, Marcel A Müller 10, Christian Drosten 3, Stefan Pöhlmann 11
Affiliations
- PMID: 32142651
- PMCID: PMC7102627
- DOI: 10.1016/j.cell.2020.02.052
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
Markus Hoffmann et al. Cell. 2020.
Abstract
The recent emergence of the novel, pathogenic SARS-coronavirus 2 (SARS-CoV-2) in China and its rapid national and international spread pose a global health emergency. Cell entry of coronaviruses depends on binding of the viral spike (S) proteins to cellular receptors and on S protein priming by host cell proteases. Unravelling which cellular factors are used by SARS-CoV-2 for entry might provide insights into viral transmission and reveal therapeutic targets. Here, we demonstrate that SARS-CoV-2 uses the SARS-CoV receptor ACE2 for entry and the serine protease TMPRSS2 for S protein priming. A TMPRSS2 inhibitor approved for clinical use blocked entry and might constitute a treatment option. Finally, we show that the sera from convalescent SARS patients cross-neutralized SARS-2-S-driven entry. Our results reveal important commonalities between SARS-CoV-2 and SARS-CoV infection and identify a potential target for antiviral intervention.
Keywords: ACE2; COVID-19; SARS-CoV-2; TMPRSS2; coronavirus; entry; neutralization; priming; spike.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures
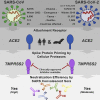
Graphical abstract
Figure 1
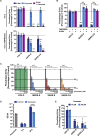
SARS-2-S and SARS-S Facilitate Entry into a Similar Panel of Mammalian Cell Lines (A) Schematic illustration of SARS-S including functional domains (RBD, receptor binding domain; RBM, receptor binding motif; TD, transmembrane domain) and proteolytic cleavage sites (S1/S2, S2′). Amino acid sequences around the two protease recognition sites (red) are indicated for SARS-S and SARS-2-S (asterisks indicate conserved residues). Arrow heads indicate the cleavage site. (B) Analysis of SARS-2-S expression (upper panel) and pseudotype incorporation (lower panel) by western blot using an antibody directed against the C-terminal hemagglutinin (HA) tag added to the viral S proteins analyzed. Shown are representative blots from three experiments. β-Actin (cell lysates) and VSV-M (particles) served as loading controls (M, matrix protein). Black arrow heads indicate bands corresponding to uncleaved S proteins (S0) whereas gray arrow heads indicate bands corresponding to the S2 subunit. (C) Cell lines of human and animal origin were inoculated with pseudotyped VSV harboring VSV-G, SARS-S, or SARS-2-S. At 16 h postinoculation, pseudotype entry was analyzed by determining luciferase activity in cell lysates. Signals obtained for particles bearing no envelope protein were used for normalization. The average of three independent experiments is shown. Error bars indicate SEM. Unprocessed data from a single experiment are presented in Figure S1.
Figure S1
Representative Experiment Included in the Average, Related to Figure 1C The indicated cells lines were inoculated with pseudoparticles harboring the indicated viral glycoprotein or harboring no glycoprotein (no protein) and luciferase activities in cell lysates were determined at 16 h posttransduction. The experiment was performed with quadruplicate samples, the average ± SD is shown.
Figure 2
SARS-2-S Harbors Amino Acid Residues Critical for ACE2 Binding (A) The S protein of SARS-CoV-2 clusters phylogenetically with S proteins of known bat-associated betacoronaviruses (see Figure S2 for more details). (B) Alignment of the receptor binding motif of SARS-S with corresponding sequences of bat-associated betacoronavirus S proteins, which are able or unable to use ACE2 as cellular receptor, reveals that SARS-CoV-2 possesses crucial amino acid residues for ACE2 binding.
Figure S2
Extended Version of the Phylogenetic Tree, Related to Figure 2B
Figure 3
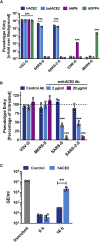
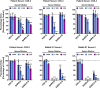
SARS-2-S Utilizes ACE2 as Cellular Receptor (A) BHK-21 cells transiently expressing ACE2 of human or bat origin, human APN, or human DPP4 were inoculated with pseudotyped VSV harboring VSV-G, SARS-S, SARS-2-S, MERS-S, or 229E-S. At 16 h postinoculation, pseudotype entry was analyzed (normalization against particles without viral envelope protein). (B) Untreated Vero cells as well as Vero cells pre-incubated with 2 or 20 μg/mL of anti-ACE2 antibody or unrelated control antibody (anti-DC-SIGN, 20 μg/mL) were inoculated with pseudotyped VSV harboring VSV-G, SARS-S, SARS-2-S, or MERS-S. At 16 h postinoculation, pseudotype entry was analyzed (normalization against untreated cells). (C) BHK-21 cells transfected with ACE2-encoding plasmid or control transfected with DsRed-encoding plasmid were infected with SARS-CoV-2 and washed, and genome equivalents in culture supernatants were determined by quantitative RT-PCR. The average of three independent experiments conducted with triplicate samples is shown in (A–C). Error bars indicate SEM. Statistical significance was tested by two-way ANOVA with Dunnett posttest. Cells transfected with empty vector served as reference in (A) whereas cells that were not treated with antibody served as reference in (B).
Figure S3
Protease Requirement for SARS-2-S-Driven Entry and Absence of Unwanted Cytotoxicity of Camostat Mesylate, Related to Figure 4 (A and B) Importance of endosomal low pH (A) and activity of CatB/L or TMPRSS2 (B) for host cell entry of SARS-CoV-2 was evaluated by adding inhibitors to target cells prior to transduction. Ammonium chloride (A) blocks endosomal acidification while E-64d and camostat mesylate (B) block the activity of CatB/L and TMPRSS2, respectively. Entry into cells not treated with inhibitor was set as 100%. (C) Absence of cytotoxic effects of camostat mesylate. Calu-3 cells were treated with camostat mesylate identically as for infection experiments and cell viability was measured using a commercially available assay (CellTiter-Glo, Promega).
Figure 4
SARS-2-S Employs TMPRSS2 for S Protein Priming in Human Lung Cells (A) Importance of activity of CatB/L or TMPRSS2 for host cell entry of SARS-CoV-2 was evaluated by adding inhibitors to target cells prior to transduction. E-64d and camostat mesylate block the activity of CatB/L and TMPRSS2, respectively (additional data for 293T cells transiently expressing ACE2 and Caco-2 cells are shown in Figure S3). (B) To analyze whether TMPRSS2 can rescue SARS-2-S-driven entry into cells that have low CatB/L activity, 293T cells transiently expressing ACE2 alone or in combination with TMPRSS2 were incubated with CatB/L inhibitor E-64d or DMSO as control and inoculated with pseudotypes bearing the indicated viral surface proteins. (C) Calu-3 cells were pre-incubated with the indicated concentrations of camostat mesylate and subsequently inoculated with pseudoparticles harboring the indicated viral glycoproteins. (D) Calu-3 cells were pre-incubated with camostat mesylate and infected with SARS-CoV-2. Subsequently, the cells were washed and genome equivalents in culture supernatants were determined by quantitative RT-PCR. (E) In order to investigate whether serine protease activity is required for SARS-2-S-driven entry into human lung cells, primary human airway epithelial cells were incubated with camostat mesylate prior to transduction. The average of three independent experiments conducted with triplicate or quadruplicate samples is shown in (A–E). Error bars indicate SEM. Statistical significance was tested by two-way ANOVA with Dunnett posttest. Cells that did not receive inhibitor served as reference in (A), (C), (D), and (E) whereas cells transfected with empty vector and not treated with inhibitor served as reference in (B).
Figure 5
Sera from Convalescent SARS Patients Cross-Neutralize SARS-2-S-Driven Entry Pseudotypes harboring the indicated viral surface proteins were incubated with different dilutions of sera from three convalescent SARS patients or sera from rabbits immunized with the S1 subunit of SARS-S and subsequently inoculated onto Vero cells in order to evaluate cross-neutralization potential. The average of three independent experiments performed with triplicate samples is shown. Error bars indicate SEM. Statistical significance was tested by two-way ANOVA with Dunnett posttest.
Comment in
- Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19.
Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S. Hoffmann M, et al. Antimicrob Agents Chemother. 2020 May 21;64(6):e00754-20. doi: 10.1128/AAC.00754-20. Print 2020 May 21. Antimicrob Agents Chemother. 2020. PMID: 32312781 Free PMC article. No abstract available. - COVID-19: A New Virus, but a Familiar Receptor and Cytokine Release Syndrome.
Hirano T, Murakami M. Hirano T, et al. Immunity. 2020 May 19;52(5):731-733. doi: 10.1016/j.immuni.2020.04.003. Epub 2020 Apr 22. Immunity. 2020. PMID: 32325025 Free PMC article. - Repurposing the mucolytic cough suppressant and TMPRSS2 protease inhibitor bromhexine for the prevention and management of SARS-CoV-2 infection.
Maggio R, Corsini GU. Maggio R, et al. Pharmacol Res. 2020 Jul;157:104837. doi: 10.1016/j.phrs.2020.104837. Epub 2020 Apr 22. Pharmacol Res. 2020. PMID: 32334052 Free PMC article. No abstract available. - Should we try the antiinflammatory natural product, celastrol, for COVID-19?
Habtemariam S, Nabavi SF, Berindan-Neagoe I, Cismaru CA, Izadi M, Sureda A, Nabavi SM. Habtemariam S, et al. Phytother Res. 2020 Jun;34(6):1189-1190. doi: 10.1002/ptr.6711. Epub 2020 Apr 29. Phytother Res. 2020. PMID: 32347602 Free PMC article. No abstract available. - Targeting the entry step of SARS-CoV-2: a promising therapeutic approach.
Li J, Zhan P, Liu X. Li J, et al. Signal Transduct Target Ther. 2020 Jun 17;5(1):98. doi: 10.1038/s41392-020-0195-x. Signal Transduct Target Ther. 2020. PMID: 32555145 Free PMC article. No abstract available. - Commentary: SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor.
Djomkam ALZ, Olwal CO, Sala TB, Paemka L. Djomkam ALZ, et al. Front Oncol. 2020 Aug 19;10:1448. doi: 10.3389/fonc.2020.01448. eCollection 2020. Front Oncol. 2020. PMID: 32974166 Free PMC article. No abstract available.
Similar articles
- The Anticoagulant Nafamostat Potently Inhibits SARS-CoV-2 S Protein-Mediated Fusion in a Cell Fusion Assay System and Viral Infection In Vitro in a Cell-Type-Dependent Manner.
Yamamoto M, Kiso M, Sakai-Tagawa Y, Iwatsuki-Horimoto K, Imai M, Takeda M, Kinoshita N, Ohmagari N, Gohda J, Semba K, Matsuda Z, Kawaguchi Y, Kawaoka Y, Inoue JI. Yamamoto M, et al. Viruses. 2020 Jun 10;12(6):629. doi: 10.3390/v12060629. Viruses. 2020. PMID: 32532094 Free PMC article. - Optimized Pseudotyping Conditions for the SARS-COV-2 Spike Glycoprotein.
Johnson MC, Lyddon TD, Suarez R, Salcedo B, LePique M, Graham M, Ricana C, Robinson C, Ritter DG. Johnson MC, et al. J Virol. 2020 Oct 14;94(21):e01062-20. doi: 10.1128/JVI.01062-20. Print 2020 Oct 14. J Virol. 2020. PMID: 32788194 Free PMC article. - TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein.
Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. Heurich A, et al. J Virol. 2014 Jan;88(2):1293-307. doi: 10.1128/JVI.02202-13. Epub 2013 Nov 13. J Virol. 2014. PMID: 24227843 Free PMC article. - Potential therapeutic targets and promising drugs for combating SARS-CoV-2.
Zhou H, Fang Y, Xu T, Ni WJ, Shen AZ, Meng XM. Zhou H, et al. Br J Pharmacol. 2020 Jul;177(14):3147-3161. doi: 10.1111/bph.15092. Epub 2020 Jun 5. Br J Pharmacol. 2020. PMID: 32368792 Free PMC article. Review. - SARS-CoV-2 pandemic and research gaps: Understanding SARS-CoV-2 interaction with the ACE2 receptor and implications for therapy.
Datta PK, Liu F, Fischer T, Rappaport J, Qin X. Datta PK, et al. Theranostics. 2020 Jun 12;10(16):7448-7464. doi: 10.7150/thno.48076. eCollection 2020. Theranostics. 2020. PMID: 32642005 Free PMC article. Review.
Cited by
- Can the use of iron phthalocyanine-derivative mouthrinses in COVID-19 patients provide systemic benefits? Research into this potential should be considered.
da Fonseca Orcina B, Bertin L, Izu Nakamura Pietro EC, Pescinelli Garcia Kuroda J, Marques da Costa Alves L, Vieira Vilhena F, da Silva Santos PS. da Fonseca Orcina B, et al. GMS Hyg Infect Control. 2024 Oct 2;19:Doc45. doi: 10.3205/dgkh000500. eCollection 2024. GMS Hyg Infect Control. 2024. PMID: 39553294 Free PMC article. - A cross-sectional study of fundus lesion characteristics in patients with acute visual impairment caused by COVID-19 infection.
Wei J, Chen X, Wang L, Wang Z, Li J, Wang Z. Wei J, et al. Sci Rep. 2024 Nov 15;14(1):28134. doi: 10.1038/s41598-024-79509-6. Sci Rep. 2024. PMID: 39548221 Free PMC article. - Olfactory Epithelium Infection by SARS-CoV-2: Possible Neuroinflammatory Consequences of COVID-19.
Gutiérrez-García AG, Contreras CM. Gutiérrez-García AG, et al. Complex Psychiatry. 2024 Oct 15;10(1-4):59-70. doi: 10.1159/000540982. eCollection 2024 Jan-Dec. Complex Psychiatry. 2024. PMID: 39545135 Review. - Brain-wide alterations revealed by spatial transcriptomics and proteomics in COVID-19 infection.
Zhang T, Li Y, Pan L, Sha J, Bailey M, Faure-Kumar E, Williams CK, Wohlschlegel J, Magaki S, Niu C, Lee Y, Su YC, Li X, Vinters HV, Geschwind DH. Zhang T, et al. Nat Aging. 2024 Nov;4(11):1598-1618. doi: 10.1038/s43587-024-00730-z. Epub 2024 Nov 14. Nat Aging. 2024. PMID: 39543407 - Predicting susceptibility to COVID-19 infection in patients on maintenance hemodialysis by cross-coupling soluble ACE2 concentration with lymphocyte count: an algorithmic approach.
Yuan S, Meng F, Zhou S, Liu X, Liu X, Zhang L, Wang T. Yuan S, et al. Front Med (Lausanne). 2024 Oct 30;11:1444719. doi: 10.3389/fmed.2024.1444719. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39540040 Free PMC article.
References
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pöhlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE. 2012;7:e35876. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous