Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2 - PubMed (original) (raw)
. 2020 May 14;181(4):905-913.e7.
doi: 10.1016/j.cell.2020.04.004. Epub 2020 Apr 24.
Hyesoo Kwon 2, Patricia Prado 3, Astrid Hagelkrüys 4, Reiner A Wimmer 4, Martin Stahl 5, Alexandra Leopoldi 4, Elena Garreta 3, Carmen Hurtado Del Pozo 3, Felipe Prosper 6, Juan Pablo Romero 6, Gerald Wirnsberger 7, Haibo Zhang 8, Arthur S Slutsky 8, Ryan Conder 5, Nuria Montserrat 9, Ali Mirazimi 10, Josef M Penninger 11
Affiliations
- PMID: 32333836
- PMCID: PMC7181998
- DOI: 10.1016/j.cell.2020.04.004
Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2
Vanessa Monteil et al. Cell. 2020.
Abstract
We have previously provided the first genetic evidence that angiotensin converting enzyme 2 (ACE2) is the critical receptor for severe acute respiratory syndrome coronavirus (SARS-CoV), and ACE2 protects the lung from injury, providing a molecular explanation for the severe lung failure and death due to SARS-CoV infections. ACE2 has now also been identified as a key receptor for SARS-CoV-2 infections, and it has been proposed that inhibiting this interaction might be used in treating patients with COVID-19. However, it is not known whether human recombinant soluble ACE2 (hrsACE2) blocks growth of SARS-CoV-2. Here, we show that clinical grade hrsACE2 reduced SARS-CoV-2 recovery from Vero cells by a factor of 1,000-5,000. An equivalent mouse rsACE2 had no effect. We also show that SARS-CoV-2 can directly infect engineered human blood vessel organoids and human kidney organoids, which can be inhibited by hrsACE2. These data demonstrate that hrsACE2 can significantly block early stages of SARS-CoV-2 infections.
Keywords: COVID-19; angiotensin converting enzyme 2; blood vessels; human organoids; kidney; severe acute respiratory syndrome coronavirus; spike glycoproteins; treatment.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests J.M.P. declares a conflict of interest as a founder, supervisory board member, and shareholder of Apeiron Biologics. G.W. is an employee of Apeiron Biologics. Apeiron holds a patent on the use of ACE2 for the treatment of lung, heart, or kidney injury and applied for a patent to treat COVID-19 with hrsACE2 and use organoids to test new drugs for SARS-CoV-2 infections. R.C. and M.S. are employees of STEMCELL Technologies Inc. A.S.S. has been a consultant to Apeiron Biologics. All other authors declare no competing interests.
Figures
Graphical abstract
Figure 1
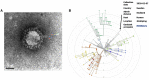
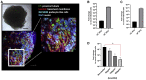
SARS-CoV-2 Sweden Virus Analyses (A) Electron microscopy image of a viral particle of the Swedish SARS-CoV-2 isolate. (B) Phylogenetic tree mapping the Swedish SARS-CoV-2 to clade A3.
Figure 2
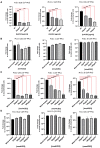
Human Recombinant Soluble ACE2 (hrsACE2) Blocks SARS-CoV-2 Infections (A) Different concentrations of human recombinant ACE2 (hrsACE2) were mixed with SARS-CoV-2 for 30 min and then added to the culture medium of Vero-E6 cells. Cells were washed after 1 h post-infection (hpi) and incubated with fresh medium. Cell were recovered 15 hpi, and viral RNA was assayed by qRT-PCR. Data are represented as mean ± SD. (Student’s t test:∗∗p < 0.01; ∗∗∗p < 0.001). (B) Murine recombinant soluble ACE2 (mrsACE2) did not significantly affect SARS-CoV-2 infections of Vero-E6 cells, highlighting the specificity of hrsACE2 in blocking SARS-CoV-2 entry. mrsACE2 was mixed with SARS-CoV-2 for 30 min and then added to the culture medium of Vero E6 cells. Cells were washed after 1 hpi and incubated with fresh medium. Cells were recovered 15 hpi, and viral RNA was assayed by qRT-PCR. Data are represented as mean ± SD. (C) Effect of hrsACE2 treatment on progeny virus. Vero E6 cells were infected with the indicated MOI of SARS-CoV-2, (the inoculum was not removed). Cells were recovered 15 hpi and viral RNA was assayed by qRT-PCR. Inhibition of the progeny virus by hsrACE2 resulted in significantly reduced virus infections. Data are represented as mean ± SD (Student’s t test: ∗p < 0.05; ∗∗p < 0.01). (D) Murine recombinant soluble ACE2 (mrsACE2) did not significantly affect SARS-CoV-2 infections of Vero-E6 cells, highlighting the specificity of hsrACE2 in blocking SARS-CoV-2 entry. Vero-E6 cells were infected with the indicated MOI of SARS-CoV-2 treated with murine recombinant soluble ACE2. Cells were harvested at 15 hpi, and viral RNA was assayed by qRT-PCR.
Figure 3
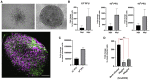
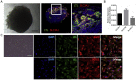
SARS-CoV-2 Infections of Blood Vessels Organoids (A) Representative images of vascular capillary organoids using light microscopy (magnifications ×10) (upper panels) and immunostaining of blood vessel organoids using anti-CD31 to detect endothelial cells and anti-PDGFRβ to detect pericytes. DAPI (blue) was used to visualize nuclei. Scale bars, 500 μm and 50 μm (inset). (B) Recovery of viral RNA from blood vessel organoids at day 3 and 6 post-infection (dpi) with SARS-CoV-2, demonstrating that the virus can infect the vascular organoids. Data are represented as mean ± SD. (C) Determination of progeny virus. Supernatants of SARS-CoV-2 infected blood vessel organoids were collected 6 dpi and then used to infect Vero E6 cells. After 48 h, Vero E6 cells were washed and viral RNA assessed by qRT-PCR. The data show that infected blood vessel organoids can produce progeny SARS-CoV-2 viruses, depending on the initial level of infection. Data are represented as mean ± SD. (D) Effect of hrsACE2 on SARS-CoV-2 infections of blood vessel organoids. Organoids were infected with a mix of 106 infectious viral particles and hrsACE2 for 1 h. 3 dpi, levels of viral RNA were assessed by qRT-PCR. hrsACE2 significantly decreased the level of SARS-CoV-2 infections in the vascular organoids. Data are represented as mean ± SD (Student’s t test: ∗∗p < 0.01).
Figure 4
SARS-CoV-2 Infections of Human Kidney Organoids (A) Representative images of a kidney organoid at day 20 of differentiation visualized using light microscopy (top left inset; scale bar, 100 μm) and confocal microscopy. Confocal microscopy images show tubular-like structures labeled with Lotus tetraglobus lectin (LTL, in green) and podocyte-like cells showing positive staining for nephrin (in turquoise). Laminin (in red) was used as a basement membrane marker. DAPI labels nuclei. A magnified view of the boxed region shows a detail of tubular structures. Scale bars, 250 and 100 μm, respectively. (B) Recovery of viral RNA in the kidney organoids at day 6 dpi with SARS-CoV-2. Data are represented as mean ± SD. (C) Determination of progeny virus. Supernatants of SARS-CoV-2 infected kidney organoids were collected 6 dpi and then used to infect Vero E6 cells. After 48 h, Vero E6 cells were washed and viral RNA assessed by qRT-PCR. The data show that infected kidney organoids can produce progeny SARS-CoV-2 viruses, depending on the initial level of infection. Data are represented as mean ± SD. (D) Effect of hrsACE2 on SARS-CoV-2 infections kidney organoids. Organoids were infected with a mix of 106 infectious viral particles and hrsACE2 for 1 h. 3 dpi, levels of viral RNA were assessed by qRT-PCR. hrsACE2 significantly decreased the level of SARS-CoV-2 infections in the kidney organoids. Data are represented as mean ± SD (Student’s t test: ∗p < 0.05).
Figure S1
Human Kidney Organoids as a Surrogate of Human Proximal Tubule Cell Culture Model, Related to Figure 4 (A) Left image corresponds to a kidney organoid at day 20 of differentiation visualized using light microscopy. Scale bar 100 μm. Confocal microscopy images of tubular-like structures labeled with Lotus Tetraglobus Lectin (LTL, in green) and the proximal tubular cell marker SCL3A1 (in red). DAPI labels nuclei. A magnified view of the boxed region shows a detail of the tubular structures. Scale bars 250 and 50 μm, respectively. (B) Expression changes of SLC3A1, SLC5A12 and SLC27A2 of bulk samples at day 20 of organoid differentiation. (C) Left image corresponds to LTL+ cells visualized using light microscopy. Scale bar 100 μm. Confocal microscopy images of LTL+ cells labeled with Lotus Tetraglobus Lectin (LTL, in green) and the proximal tubular cell markers NaK ATPase (NaK, in red) and the solute carrier SGLT2 (in red). DAPI was used to visualize nuclei. Scale bars 100 μm.
Figure S2
Single-Cell RNA-Seq Analysis of Kidney Organoids Reveals ACE2 Expression in Proximal Tubule Cells, Related to Figure 4 (A) UMAP plot displaying the results after unbiased clustering. Subpopulations of renal endothelial-like, mesenchymal, proliferating, podocyte and tubule cells were identified. (B) Expression of ACE2 projected in the UMAP reduction. (C) Expression of different cellular markers: SLC3A1, SLC27A2 (Proximal Tubule); PODXL, NPHS1, NPHS2 (Podocyte); CLDN4, MAL (Loop of Henle) and CD93 (Renal Endothelial-like cells).
Comment in
- Blockade of SARS-CoV-2 infection by recombinant soluble ACE2.
Alhenc-Gelas F, Drueke TB. Alhenc-Gelas F, et al. Kidney Int. 2020 Jun;97(6):1091-1093. doi: 10.1016/j.kint.2020.04.009. Epub 2020 Apr 14. Kidney Int. 2020. PMID: 32354636 Free PMC article. No abstract available.
Similar articles
- Expressions and significances of the angiotensin-converting enzyme 2 gene, the receptor of SARS-CoV-2 for COVID-19.
Fu J, Zhou B, Zhang L, Balaji KS, Wei C, Liu X, Chen H, Peng J, Fu J. Fu J, et al. Mol Biol Rep. 2020 Jun;47(6):4383-4392. doi: 10.1007/s11033-020-05478-4. Epub 2020 May 14. Mol Biol Rep. 2020. PMID: 32410141 Free PMC article. - Organ-protective effect of angiotensin-converting enzyme 2 and its effect on the prognosis of COVID-19.
Cheng H, Wang Y, Wang GQ. Cheng H, et al. J Med Virol. 2020 Jul;92(7):726-730. doi: 10.1002/jmv.25785. Epub 2020 Apr 5. J Med Virol. 2020. PMID: 32221983 Free PMC article. Review. - Antiviral Activity of Type I, II, and III Interferons Counterbalances ACE2 Inducibility and Restricts SARS-CoV-2.
Busnadiego I, Fernbach S, Pohl MO, Karakus U, Huber M, Trkola A, Stertz S, Hale BG. Busnadiego I, et al. mBio. 2020 Sep 10;11(5):e01928-20. doi: 10.1128/mBio.01928-20. mBio. 2020. PMID: 32913009 Free PMC article. - A brief review of interplay between vitamin D and angiotensin-converting enzyme 2: Implications for a potential treatment for COVID-19.
Malek Mahdavi A. Malek Mahdavi A. Rev Med Virol. 2020 Sep;30(5):e2119. doi: 10.1002/rmv.2119. Epub 2020 Jun 25. Rev Med Virol. 2020. PMID: 32584474 Free PMC article. Review. - Could a specific ACE2 activator drug improve the clinical outcome of SARS-CoV-2? A potential pharmacological insight.
Nicolau LAD, Nolêto IRSG, Medeiros JVR. Nicolau LAD, et al. Expert Rev Clin Pharmacol. 2020 Aug;13(8):807-811. doi: 10.1080/17512433.2020.1798760. Epub 2020 Jul 25. Expert Rev Clin Pharmacol. 2020. PMID: 32686527 Free PMC article. No abstract available.
Cited by
- Digestive system involvement of infections with SARS-CoV-2 and other coronaviruses: Clinical manifestations and potential mechanisms.
Zhan GF, Wang Y, Yang N, Luo AL, Li SY. Zhan GF, et al. World J Gastroenterol. 2021 Feb 21;27(7):561-575. doi: 10.3748/wjg.v27.i7.561. World J Gastroenterol. 2021. PMID: 33642829 Free PMC article. Review. - Reply to Liu et al.: Specific mutations matter in specificity and catalysis in ACE2.
Glasgow J, Glasgow A, Kortemme T, Wells JA. Glasgow J, et al. Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2024450118. doi: 10.1073/pnas.2024450118. Proc Natl Acad Sci U S A. 2021. PMID: 33833059 Free PMC article. No abstract available. - CD38 in the age of COVID-19: a medical perspective.
Horenstein AL, Faini AC, Malavasi F. Horenstein AL, et al. Physiol Rev. 2021 Oct 1;101(4):1457-1486. doi: 10.1152/physrev.00046.2020. Epub 2021 Mar 31. Physiol Rev. 2021. PMID: 33787351 Free PMC article. Review. - Vascular Aging and COVID-19.
Badaras I, Laučytė-Cibulskienė A. Badaras I, et al. Angiology. 2023 Apr;74(4):308-316. doi: 10.1177/00033197221121007. Epub 2022 Aug 28. Angiology. 2023. PMID: 36031949 Free PMC article. Review. - Dysregulation in Akt/mTOR/HIF-1 signaling identified by proteo-transcriptomics of SARS-CoV-2 infected cells.
Appelberg S, Gupta S, Svensson Akusjärvi S, Ambikan AT, Mikaeloff F, Saccon E, Végvári Á, Benfeitas R, Sperk M, Ståhlberg M, Krishnan S, Singh K, Penninger JM, Mirazimi A, Neogi U. Appelberg S, et al. Emerg Microbes Infect. 2020 Dec;9(1):1748-1760. doi: 10.1080/22221751.2020.1799723. Emerg Microbes Infect. 2020. PMID: 32691695 Free PMC article.
References
- Bertram S., Heurich A., Lavender H., Gierer S., Danisch S., Perin P., Lucas J.M., Nelson P.S., Pöhlmann S., Soilleux E.J. Influenza and SARS-coronavirus activating proteases TMPRSS2 and HAT are expressed at multiple sites in human respiratory and gastrointestinal tracts. PLoS ONE. 2012;7:e35876. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous