Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19 - PubMed (original) (raw)
Comparative Study
. 2020 Jul 9;383(2):120-128.
doi: 10.1056/NEJMoa2015432. Epub 2020 May 21.
Stijn E Verleden 1, Mark Kuehnel 1, Axel Haverich 1, Tobias Welte 1, Florian Laenger 1, Arno Vanstapel 1, Christopher Werlein 1, Helge Stark 1, Alexandar Tzankov 1, William W Li 1, Vincent W Li 1, Steven J Mentzer 1, Danny Jonigk 1
Affiliations
- PMID: 32437596
- PMCID: PMC7412750
- DOI: 10.1056/NEJMoa2015432
Comparative Study
Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19
Maximilian Ackermann et al. N Engl J Med. 2020.
Abstract
Background: Progressive respiratory failure is the primary cause of death in the coronavirus disease 2019 (Covid-19) pandemic. Despite widespread interest in the pathophysiology of the disease, relatively little is known about the associated morphologic and molecular changes in the peripheral lung of patients who die from Covid-19.
Methods: We examined 7 lungs obtained during autopsy from patients who died from Covid-19 and compared them with 7 lungs obtained during autopsy from patients who died from acute respiratory distress syndrome (ARDS) secondary to influenza A(H1N1) infection and 10 age-matched, uninfected control lungs. The lungs were studied with the use of seven-color immunohistochemical analysis, micro-computed tomographic imaging, scanning electron microscopy, corrosion casting, and direct multiplexed measurement of gene expression.
Results: In patients who died from Covid-19-associated or influenza-associated respiratory failure, the histologic pattern in the peripheral lung was diffuse alveolar damage with perivascular T-cell infiltration. The lungs from patients with Covid-19 also showed distinctive vascular features, consisting of severe endothelial injury associated with the presence of intracellular virus and disrupted cell membranes. Histologic analysis of pulmonary vessels in patients with Covid-19 showed widespread thrombosis with microangiopathy. Alveolar capillary microthrombi were 9 times as prevalent in patients with Covid-19 as in patients with influenza (P<0.001). In lungs from patients with Covid-19, the amount of new vessel growth - predominantly through a mechanism of intussusceptive angiogenesis - was 2.7 times as high as that in the lungs from patients with influenza (P<0.001).
Conclusions: In our small series, vascular angiogenesis distinguished the pulmonary pathobiology of Covid-19 from that of equally severe influenza virus infection. The universality and clinical implications of our observations require further research to define. (Funded by the National Institutes of Health and others.).
Copyright © 2020 Massachusetts Medical Society.
Figures
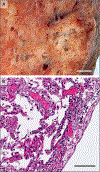
Figure 1.. Lymphocytic inflammation in a Lung from a Patient Who Died from Covid-19.
The gross appearance of a lung from a patient who died from coronavirus disease 2019 (Covid-19) is shown in Panel A (the scale bar corresponds to 1 cm). The histopathological examination, shown in Panel B, revealed interstitial and perivascular predominantly lymphocytic pneumonia with multifocal endothelialitis (hematoxylin–eosin staining; the scale bar corresponds to 200 _μ_m).
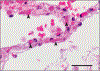
Figure 2.. Microthrombi in the interalveolar Septa of a Lung from a Patient Who Died from Covid-19.
The interalveolar septum of this patient (Patient 4 in Table S1A in the Supplementary Appendix) shows slightly expanded alveolar walls with multiple fibrinous microthrombi (arrowheads) in the alveolar capillaries. Extravasated erythrocytes and a loose network of fibrin can be seen in the intraalveolar space (hematoxylin–eosin staining; the scale bar corresponds to 50 _μ_m).
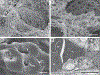
Figure 3.. Microvascular Alterations in Lungs from Patients Who Died from Covid-19.
Panels A and B show scanning electron micrographs of microvascular corrosion casts from the thin-walled alveolar plexus of a healthy lung (Panel A) and the substantial architectural distortion seen in lungs injured by Covid-19 (Panel B). The loss of a clearly visible vessel hierarchy in the alveolar plexus is the result of new blood-vessel formation by intussusceptive angiogenesis. Panel C shows the intussusceptive pillar localizations (arrowheads) at higher magnification. Panel D is a transmission electron micrograph showing ultrastructural features of endothelial cell destruction and SARS-CoV-2 visible within the cell membrane (arrowheads) (the scale bar corresponds to 5 _μ_m). RC denotes red cell.
Figure 4 (facing page).. Numeric Density of Features of intussusceptive and Sprouting Angiogenesis in Lungs from Patients Who Died from Covid-19 or influenza A(H1N1).
Angiogenic features of sprouting and intussusceptive angiogenesis (intussusceptive pillars and sprouts, respectively) were counted per field of view in microvascular corrosion casts of lungs from patients with Covid-19 (red), lungs from patients with influenza A(H1N1) (blue), and control lungs (white). In Panels A and B, the numeric densities of angiogenic features are summarized as box plots for intussusceptive and sprouting angiogenesis. The boxes reflect the interquartile range, and the whiskers indicate the range (up to 1.5 times the interquartile range). Outliers are denoted by singular points. A statistical comparison between lungs from patients Covid-19 and those from patients with influenza and uninfected control lungs showed a significantly higher frequency of angiogenesis in the patients with Covid-19 lungs, especially intussusceptive angiogenesis (P values were calculated with Student’s t-test, controlled for the familywise error rate with a Benjamini–Hochberg false discovery rate threshold of 0.05). Panels C and D show a chronological comparison of intussusceptive and sprouting angiogenesis in lungs from patients with Covid-19 and lungs from patients with influenza A(H1N1) plotted as a function of the duration of hospitalization. The numbers shown are Pearson correlation coefficients and P values (all displayed P values of 0.05 or lower also pass the false discovery rate threshold of 0.05). The median angiogenic feature count for each patient is displayed as one dot. The shaded areas encompassing the dotted linear regression lines are smoothed 95% confidence intervals. As a reference for increased blood-vessel formation in lung diseases, intussusceptive and sprouting angiogenesis as found in end-stage nonspecific interstitial pneumonia (NSIP), a chronic interstitial lung disease, at the time of lung transplantation (a mean of 1650 days from first consultation to lung transplantation) are shown (purple box plots). Findings in healthy control lungs are also indicated (white box plots). The white and purple box plots are displayed in relation to the y axis but not the x axis (as indicated by vertical dashed lines).
Figure 5.. Relative Expression Analysis of Angiogenesis-Associated Genes in Lungs from Patients Who Died from Covid-19 or Influenza A(H1N1).
RNA was isolated from sections sampled directly adjacent to those used for complementary histologic and immunohistochemical analyses. RNA was isolated with the Maxwell RNA extraction system (Promega) and, after quality control through Qubit analysis (ThermoFisher), was used for further analysis. During the NanoString procedure, individual copies of all RNA molecules were labeled with gene-specific bar codes and counted individually with the nCounter Analysis System (NanoString Technologies). The expression of angiogenesis-associated genes was measured with the NanoString nCounter PanCancer Progression panel (323 target genes annotated as relevant for angiogenesis). The resulting gene-expression data were normalized to negative control lanes (arithmetic mean background subtraction), positive control lanes (geometric mean normalization factor), and all reference genes present on the panel (geometric mean normalization factor) with the use of nSolver Analysis Software, version 4.0. Shown in the Venn diagram are only genes that are statistically differentially expressed as compared with expression in controls in both disease groups (Student’s t-test, controlled for the familywise error rate with a Benjamini–Hochberg false discovery rate threshold of 0.05). Up-regulation and down-regulation of genes is indicated by colored arrowheads suffixed to the gene symbols (purple denotes up-regulation, red denotes down-regulation).
Comment in
- Covid-19, Angiogenesis, and ARDS Endotypes.
Hariri L, Hardin CC. Hariri L, et al. N Engl J Med. 2020 Jul 9;383(2):182-183. doi: 10.1056/NEJMe2018629. Epub 2020 May 21. N Engl J Med. 2020. PMID: 32437597 No abstract available. - Pericyte alteration sheds light on micro-vasculopathy in COVID-19 infection.
Cardot-Leccia N, Hubiche T, Dellamonica J, Burel-Vandenbos F, Passeron T. Cardot-Leccia N, et al. Intensive Care Med. 2020 Sep;46(9):1777-1778. doi: 10.1007/s00134-020-06147-7. Epub 2020 Jun 12. Intensive Care Med. 2020. PMID: 32533198 Free PMC article. No abstract available. - Pulmonary Vascular Pathology in Covid-19.
Burel-Vandenbos F, Cardot-Leccia N, Passeron T. Burel-Vandenbos F, et al. N Engl J Med. 2020 Aug 27;383(9):886-887. doi: 10.1056/NEJMc2022068. Epub 2020 Jul 17. N Engl J Med. 2020. PMID: 32678531 No abstract available. - Pulmonary Vascular Pathology in Covid-19.
Som A, Lang M, Little B. Som A, et al. N Engl J Med. 2020 Aug 27;383(9):887. doi: 10.1056/NEJMc2022068. Epub 2020 Jul 17. N Engl J Med. 2020. PMID: 32678532 No abstract available. - Pulmonary Vascular Pathology in Covid-19.
Scholkmann F, Nicholls J. Scholkmann F, et al. N Engl J Med. 2020 Aug 27;383(9):887-888. doi: 10.1056/NEJMc2022068. Epub 2020 Jul 17. N Engl J Med. 2020. PMID: 32678533 No abstract available. - In vivo demonstration of pulmonary microvascular involvement in COVID-19 using dual-energy computed tomography.
Si-Mohamed S, Chebib N, Sigovan M, Zumbihl L, Turquier S, Boccalini S, Boussel L, Mornex JF, Cottin V, Douek P. Si-Mohamed S, et al. Eur Respir J. 2020 Oct 29;56(4):2002608. doi: 10.1183/13993003.02608-2020. Print 2020 Oct. Eur Respir J. 2020. PMID: 32943402 Free PMC article. - Pulmonary vascular proliferation in patients with severe COVID-19: an autopsy study.
Pérez-Mies B, Gómez-Rojo M, Carretero-Barrio I, Bardi T, Benito A, García-Cosío M, Caballero Á, de Pablo R, Galán JC, Pestaña D, Palacios J. Pérez-Mies B, et al. Thorax. 2021 Oct;76(10):1044-1046. doi: 10.1136/thoraxjnl-2020-216714. Epub 2021 Mar 23. Thorax. 2021. PMID: 33758071 Free PMC article.
Similar articles
- Diffuse alveolar damage (DAD) resulting from coronavirus disease 2019 Infection is Morphologically Indistinguishable from Other Causes of DAD.
Konopka KE, Nguyen T, Jentzen JM, Rayes O, Schmidt CJ, Wilson AM, Farver CF, Myers JL. Konopka KE, et al. Histopathology. 2020 Oct;77(4):570-578. doi: 10.1111/his.14180. Epub 2020 Sep 12. Histopathology. 2020. PMID: 32542743 Free PMC article. - COVID-19 pulmonary pathology: a multi-institutional autopsy cohort from Italy and New York City.
Borczuk AC, Salvatore SP, Seshan SV, Patel SS, Bussel JB, Mostyka M, Elsoukkary S, He B, Del Vecchio C, Fortarezza F, Pezzuto F, Navalesi P, Crisanti A, Fowkes ME, Bryce CH, Calabrese F, Beasley MB. Borczuk AC, et al. Mod Pathol. 2020 Nov;33(11):2156-2168. doi: 10.1038/s41379-020-00661-1. Epub 2020 Sep 2. Mod Pathol. 2020. PMID: 32879413 Free PMC article. - Lung Histopathology in Coronavirus Disease 2019 as Compared With Severe Acute Respiratory Sydrome and H1N1 Influenza: A Systematic Review.
Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, Vinarsky V, Rubin J, Okin DA, Sclafani A, Alladina JW, Griffith JW, Gillette MA, Raz Y, Richards CJ, Wong AK, Ly A, Hung YP, Chivukula RR, Petri CR, Calhoun TF, Brenner LN, Hibbert KA, Medoff BD, Hardin CC, Stone JR, Mino-Kenudson M. Hariri LP, et al. Chest. 2021 Jan;159(1):73-84. doi: 10.1016/j.chest.2020.09.259. Epub 2020 Oct 7. Chest. 2021. PMID: 33038391 Free PMC article. - The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities.
Buja LM, Wolf DA, Zhao B, Akkanti B, McDonald M, Lelenwa L, Reilly N, Ottaviani G, Elghetany MT, Trujillo DO, Aisenberg GM, Madjid M, Kar B. Buja LM, et al. Cardiovasc Pathol. 2020 Sep-Oct;48:107233. doi: 10.1016/j.carpath.2020.107233. Epub 2020 May 7. Cardiovasc Pathol. 2020. PMID: 32434133 Free PMC article. - [COVID-19: effects on the lungs and heart].
Ackermann M, Werlein C, Länger F, Kühnel MP, Jonigk DD. Ackermann M, et al. Pathologe. 2021 Mar;42(2):164-171. doi: 10.1007/s00292-021-00918-9. Epub 2021 Feb 9. Pathologe. 2021. PMID: 33560456 Free PMC article. Review. German.
Cited by
- Pathological Changes in the Lungs of Patients with a Lethal COVID-19 Clinical Course.
Viksne V, Strumfa I, Sperga M, Ziemelis J, Abolins J. Viksne V, et al. Diagnostics (Basel). 2022 Nov 15;12(11):2808. doi: 10.3390/diagnostics12112808. Diagnostics (Basel). 2022. PMID: 36428868 Free PMC article. - Severe Acute Respiratory Syndrome-Associated Coronavirus 2 Infection and Organ Dysfunction in the ICU: Opportunities for Translational Research.
Verhoef PA, Kannan S, Sturgill JL, Tucker EW, Morris PE, Miller AC, Sexton TR, Koyner JL, Hejal R, Brakenridge SC, Moldawer LL, Hotchkiss RS, Blood TM, Mazer MB, Bolesta S, Alexander SA, Armaignac DL, Shein SL, Jones C, Hoemann CD, Doctor A, Friess SH, Parker RI, Rotta AT, Remy KE. Verhoef PA, et al. Crit Care Explor. 2021 Mar 12;3(3):e0374. doi: 10.1097/CCE.0000000000000374. eCollection 2021 Mar. Crit Care Explor. 2021. PMID: 33786450 Free PMC article. Review. - Venous thromboembolism in COVID-19 compared to non-COVID-19 cohorts: A systematic review with meta-analysis.
Mai V, Tan BK, Mainbourg S, Potus F, Cucherat M, Lega JC, Provencher S. Mai V, et al. Vascul Pharmacol. 2021 Aug;139:106882. doi: 10.1016/j.vph.2021.106882. Epub 2021 Jun 2. Vascul Pharmacol. 2021. PMID: 34087481 Free PMC article. - Fundamental protective mechanisms of face masks against droplet infections.
Kähler CJ, Hain R. Kähler CJ, et al. J Aerosol Sci. 2020 Oct;148:105617. doi: 10.1016/j.jaerosci.2020.105617. Epub 2020 Jun 28. J Aerosol Sci. 2020. PMID: 32834103 Free PMC article. - Pre-hospital antiplatelet medication use on COVID-19 disease severity.
Pan D, Ip A, Zhan S, Wasserman I, Snyder DJ, Agathis AZ, Shamapant N, Yang JY, Pai A, Mazumdar M, Poor H. Pan D, et al. Heart Lung. 2021 Sep-Oct;50(5):618-621. doi: 10.1016/j.hrtlng.2021.04.010. Epub 2021 May 27. Heart Lung. 2021. PMID: 34090177 Free PMC article.
References
- Raptis CA, Hammer MM, Short RG, et al. Chest CT and coronavirus disease (COVID-19): a critical review of the literature to date. AJR Am J Roentgenol 2020. April 16 (Epub ahead of print). - PubMed
- Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med 2017;377:562–72. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical