TGF-β Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia? - PubMed (original) (raw)
Review
. 2020 Jul 23;11(4):828-850.
doi: 10.14336/AD.2020.0222. eCollection 2020 Jul.
Affiliations
- PMID: 32765949
- PMCID: PMC7390515
- DOI: 10.14336/AD.2020.0222
Review
TGF-β Signaling: A Therapeutic Target to Reinstate Regenerative Plasticity in Vascular Dementia?
Mahesh Kandasamy et al. Aging Dis. 2020.
Abstract
Vascular dementia (VaD) is the second leading form of memory loss after Alzheimer's disease (AD). Currently, there is no cure available. The etiology, pathophysiology and clinical manifestations of VaD are extremely heterogeneous, but the impaired cerebral blood flow (CBF) represents a common denominator of VaD. The latter might be the result of atherosclerosis, amyloid angiopathy, microbleeding and micro-strokes, together causing blood-brain barrier (BBB) dysfunction and vessel leakage, collectively originating from the consequence of hypertension, one of the main risk factors for VaD. At the histopathological level, VaD displays abnormal vascular remodeling, endothelial cell death, string vessel formation, pericyte responses, fibrosis, astrogliosis, sclerosis, microglia activation, neuroinflammation, demyelination, white matter lesions, deprivation of synapses and neuronal loss. The transforming growth factor (TGF) β has been identified as one of the key molecular factors involved in the aforementioned various pathological aspects. Thus, targeting TGF-β signaling in the brain might be a promising therapeutic strategy to mitigate vascular pathology and improve cognitive functions in patients with VaD. This review revisits the recent understanding of the role of TGF-β in VaD and associated pathological hallmarks. It further explores the potential to modulate certain aspects of VaD pathology by targeting TGF-β signaling.
Keywords: BBB; TGF-β; endothelial cells; hippocampus; neural regeneration; oxidative stress; pericytes; vascular dementia.
Copyright: © 2020 Kandasamy et al.
Figures
Figure 1.
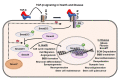
An overview of Vascular Dementia. The figure summarizes the possible biological and lifestyle risk factors, various forms of VaD, pathological hallmarks of VaD and indicates cognitive deficit as the final outcome of the disease.
Figure 2.
TGF-β signaling in health and disease. Schematic representation of the TGF-β mediated Smad signaling through the TGF-β receptors. TGF-β binds to TGF-β receptor II, which induces the activation of TGF-β receptor I and activates the phosphorylation of Smad2/3 in the cytoplasm. The activated Smad2/3 binds to Smad4 in the cytoplasm and mobilize into the nucleus where they act as a transcription factor. This leads to various cell-type dependent responses essential for the tissue homoeostasis and various physiological functions. Dysregulation of the TGF-β pathway leads to abnormal cellular events and pathological hallmarks that are all part of the VaD pathology.
Figure 3.
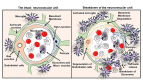
The intact neurovascular unit and breakdown of the neurovascular unit in VaD. Illustrations of the intact neurovascular unit that typically contains normal blood cells, endothelial cells, basement membrane ensheathed by pericytes and end feet of astrocytes in the brain. The figure also represents the breakdown of the neurovascular unit resulting from the BBB disruption, the crucial pathological hallmarks of VaD such as endothelial cell death and drift of pericyte. The activation of immune cells interaction between the activated microglia and peripheral blood cells leading to the release of TGF-β and breakdown of exosomes and release of macrovesicles in the brain of subjects with VaD.
Figure 4.
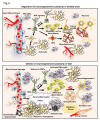
TGF-β and its involvement in neuroinflammation and neurogenesis in the control brain in the context of VaD. Illustration of the regulation of adult neurogenesis by TGF-β at the level of neural stem cell (NSC) self-renewal, transformation of neuroblasts and neuronal differentiation. The intact blood vessel, astrogenesis through astrocyte precursor cell (APS) and oligodendrogenesis from oligodendrocyte precursor cells (OPC) and resident microglial cells are indicated. In the parallel side, the figure indicates the aberrant regulation of adult neurogenesis, activated astrogliosis, microglial activation, accumulation of amyloid β, formation of string vessel, demyelination, neurodegeneration resulting from neuroinflammation mediated abnormal levels of TGF- β in VaD.
Figure 5.
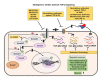
A proposed mechanism for TGF-β activation in the VaD. The figure represents the proposed hypothesis that interaction between neuroblasts and activated microglial cells might lead to the production of the inactive form of the TGF-β attached with latent transforming growth factor-β binding protein (LTBP) through latency-associated peptide (LAP). The inactive form of TGF-β has generally been stored in the extracellular matrix (ECM). Vascular damage upon mitochondrial defects, release of free radical and acidification of the local microenvironment might be underlying mechanisms of the pathological activation and release of TGF-β in VaD.
Figure 6.
Possible strategies to inactivate TGF-β signaling and expected outcomes in VaD. Illustration of possible ways to inhibit aberrant TGF-β signaling pathways. The activities of TGF-β and TGF-β receptors can be neutralized using various recombinant antibodies or their translation can be blocked using specific set of antisense oligonucleotides. Different synthetic molecules that can inhibit the phosphorylation of the TGF-β RII and Smad2/3 are also indicated. The blockade of pathogenic TGF-β signaling has been expected to mitigate the cognitive deficits through inhibition of various neuropathogenic events and promotion of neuroregenerative plasticity in VaD.
Similar articles
- Mediators of cerebral hypoperfusion and blood-brain barrier leakiness in Alzheimer's disease, vascular dementia and mixed dementia.
Tayler H, Miners JS, Güzel Ö, MacLachlan R, Love S. Tayler H, et al. Brain Pathol. 2021 Jul;31(4):e12935. doi: 10.1111/bpa.12935. Epub 2021 Feb 25. Brain Pathol. 2021. PMID: 33410232 Free PMC article. - Brain Microvascular Pericytes in Vascular Cognitive Impairment and Dementia.
Uemura MT, Maki T, Ihara M, Lee VMY, Trojanowski JQ. Uemura MT, et al. Front Aging Neurosci. 2020 Apr 14;12:80. doi: 10.3389/fnagi.2020.00080. eCollection 2020. Front Aging Neurosci. 2020. PMID: 32317958 Free PMC article. Review. - Neurovascular Alterations in Vascular Dementia: Emphasis on Risk Factors.
Lecordier S, Manrique-Castano D, El Moghrabi Y, ElAli A. Lecordier S, et al. Front Aging Neurosci. 2021 Sep 10;13:727590. doi: 10.3389/fnagi.2021.727590. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34566627 Free PMC article. - Dysfunction of Cerebrovascular Endothelial Cells: Prelude to Vascular Dementia.
Wang F, Cao Y, Ma L, Pei H, Rausch WD, Li H. Wang F, et al. Front Aging Neurosci. 2018 Nov 16;10:376. doi: 10.3389/fnagi.2018.00376. eCollection 2018. Front Aging Neurosci. 2018. PMID: 30505270 Free PMC article. Review. - Dysfunction of the blood-brain barrier in Alzheimer's disease: Evidence from human studies.
Kurz C, Walker L, Rauchmann BS, Perneczky R. Kurz C, et al. Neuropathol Appl Neurobiol. 2022 Apr;48(3):e12782. doi: 10.1111/nan.12782. Epub 2022 Feb 2. Neuropathol Appl Neurobiol. 2022. PMID: 34823269 Review.
Cited by
- SARS-CoV-2-Mediated Neuropathogenesis, Deterioration of Hippocampal Neurogenesis and Dementia.
Radhakrishnan RK, Kandasamy M. Radhakrishnan RK, et al. Am J Alzheimers Dis Other Demen. 2022 Jan-Dec;37:15333175221078418. doi: 10.1177/15333175221078418. Am J Alzheimers Dis Other Demen. 2022. PMID: 35133907 Free PMC article. - Influence of SARS-CoV-2 on Adult Human Neurogenesis.
Stępień T, Tarka S, Chmura N, Grzegorczyk M, Acewicz A, Felczak P, Wierzba-Bobrowicz T. Stępień T, et al. Cells. 2023 Jan 6;12(2):244. doi: 10.3390/cells12020244. Cells. 2023. PMID: 36672177 Free PMC article. - TGF-β1 Decreases Microglia-Mediated Neuroinflammation and Lipid Droplet Accumulation in an In Vitro Stroke Model.
Xin W, Pan Y, Wei W, Gerner ST, Huber S, Juenemann M, Butz M, Bähr M, Huttner HB, Doeppner TR. Xin W, et al. Int J Mol Sci. 2023 Dec 10;24(24):17329. doi: 10.3390/ijms242417329. Int J Mol Sci. 2023. PMID: 38139158 Free PMC article. - Physical Exercise Exerts Neuroprotective Effect on Memory Impairment by Mitigate the Decline of Striatum Catecholamine and Spine Density in a Vascular Dementia Rat Model.
Ren H, Zhang Z, Zhang J. Ren H, et al. Am J Alzheimers Dis Other Demen. 2022 Jan-Dec;37:15333175221144367. doi: 10.1177/15333175221144367. Am J Alzheimers Dis Other Demen. 2022. PMID: 36515911 Free PMC article. - Attenuation of Chronic Stress-Induced Depressive-like Symptoms by Fish Oil via Alleviating Neuroinflammation and Impaired Tryptophan Metabolism in Aging Rats.
Tung TH, Lai WD, Lee HC, Su KP, Panunggal B, Huang SY. Tung TH, et al. J Agric Food Chem. 2023 Oct 11;71(40):14550-14561. doi: 10.1021/acs.jafc.3c01784. Epub 2023 Sep 28. J Agric Food Chem. 2023. PMID: 37769277 Free PMC article.
References
- O'Brien JT, Thomas A (2015). Vascular dementia. Lancet, 386:1698-1706. - PubMed
Publication types
LinkOut - more resources
Full Text Sources