Long-Acting Injectable GLP-1 Receptor Agonists for the Treatment of Adults with Type 2 Diabetes: Perspectives from Clinical Practice - PubMed (original) (raw)
Review
. 2020 Nov 9:13:4221-4234.
doi: 10.2147/DMSO.S216054. eCollection 2020.
Affiliations
- PMID: 33204129
- PMCID: PMC7665457
- DOI: 10.2147/DMSO.S216054
Review
Long-Acting Injectable GLP-1 Receptor Agonists for the Treatment of Adults with Type 2 Diabetes: Perspectives from Clinical Practice
Mario Luca Morieri et al. Diabetes Metab Syndr Obes. 2020.
Abstract
Randomized controlled trials (RCTs) have consistently shown glycemic and extra-glycemic benefits of long-acting injectable glucagon-like-peptide-1 receptor agonists (GLP-1RAs, liraglutide, albiglutide, exenatide once-weekly, dulaglutide, and semaglutide) in terms of reduction in the rates of cardiovascular events and mortality among patients with type 2 diabetes. Recently, the analyses of large datasets collecting routinely-accumulated data from clinical practice (ie, real-world studies, RWS) have provided new opportunities to complement the information obtained from RCTs. In this narrative review, we addressed clinically relevant questions that might be answered by well-conducted RWS: are subjects treated with GLP-1RAs in the "real-world" similar to those included in RCTs? Is the performance of GLP-1RA observed in the RWS (effectiveness) similar to that described in RCTs (efficacy)? Is the effectiveness similar in population of patients generally under-represented in RCTs? Are the cardiovascular benefits of GLP-1RAs confirmed in RWS? We also describe a few comparisons currently un-explored by specific RCTs, such as direct comparison between different administration strategies (eg, fixed- versus flexible-combination with basal-insulin) or between GLP-1RAs versus dipeptidyl-peptidase-4 inhibitor (DDP4i) or versus sodium/glucose cotransporter-2 inhibitors (SGLT-2i) on hard cardio-renal outcomes. Altogether, RWS provide highly informative information on treatment with GLP-1RAs. On the one side, RWS showed different clinical characteristics between subjects enrolled in RCTs versus those attending real-world clinics and receiving a GLP-1RA. On the other hand, RWS showed that GLP-1RA effectiveness is overall consistent in subgroups of patients less represented in RCTs. In addition, RWS allowed the identification of modifiable factors (eg, titration or adherence) that might guide physicians towards better GLP-1RAs use. Finally, multiple RWS reported better cardio-renal outcomes with GLP-1RAs than with DPP-4i, while initial findings from RWS described a weaker cardiovascular protection compared to SGLT-2i. Therefore, there is the need for further RWS and RCTs comparing these different classes of glucose lowering medications.
Keywords: cardiovascular prevention; effectiveness; head-to-head comparisons; innovative; observational studies; real-world evidence.
© 2020 Morieri et al.
Conflict of interest statement
MLM received grant support, lecture or consultant fees from: Servier, Amryt and SLAPharma, not related to the submitted work. . AA received research grants, lecture or advisory board fees from: Merck Sharp & Dome, AstraZeneca, Novartis, Boehringer-Ingelheim, Sanofi, Mediolanum, Janssen, Novo Nordisk, Lilly, Servier, and Takeda, not related to the submitted work. GPF received lecture fees or grant support from: Abbott, AstraZeneca, Boehringer, Lilly, Merck-Sharp-Dome, Mundipharma, Novartis, Novo Nordisk, Sanofi, Servier, not related to the submitted work. The authors report no other potential conflicts of interest for this work.
Figures
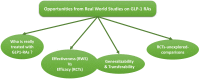
Figure 1
Opportunities from real-world studies (RWS) to implement notions from randomized controlled trials (RCTs). The figures describes some of those research questions that can be addressed with RWS, eg, the evaluation of whether subjects treated with GLP1-RAs have clinical characteristics similar to those enrolled in RCTs, or whether the benefit of GLP-1RAs described in RCTs are confirmed in RWS and in different populations (generalizability). Finally, RWS provide the opportunity to explore some head-to-head comparisons not yet tested in RCTs.
Figure 2
Lack of adequate representation in cardiovascular outcome trials (CVOTs) of subjects with type 2 diabetes attending real-world clinics. The figure describes the proportion of subjects with type 2 diabetes attending “real-world” diabetes centers that can be considered to be adequately represented in CVOTs according to two possible evaluations: the first and second from the left is according to Inclusion/Exclusion (I/E) of the trials, thethird fromthe left is according to the goal of selecting a population of subjects with clinical characteristics similar on average to those population enrolled in CVOTs.
Similar articles
- Real-World Effectiveness of Once-Weekly Glucagon-Like Peptide-1 Receptor Agonists (OW GLP-1RAs) in Comparison with Dipeptidyl Peptidase-4 Inhibitors (DPP-4is) for Glycemic Control and Weight Outcomes in Type 2 Diabetes Mellitus (RELATE).
Tan X, Divino V, Amamoo J, Xie L, Coyle KB, Gamble CL, Guevarra M, Paprocki Y, King AA. Tan X, et al. Clin Drug Investig. 2024 Apr;44(4):271-284. doi: 10.1007/s40261-024-01354-2. Epub 2024 Mar 20. Clin Drug Investig. 2024. PMID: 38507188 Free PMC article. - Protective Effects of Home T2DM Treatment with Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Co-transporter-2 Inhibitors Against Intensive Care Unit Admission and Mortality in the Acute Phase of the COVID-19 Pandemic: A Retrospective Observational Study in Italy.
Monda VM, Voci C, Strollo F, Passaro A, Greco S, Monesi M, Bigoni R, Porcellati F, Piani D, Satta E, Gentile S. Monda VM, et al. Diabetes Ther. 2023 Dec;14(12):2127-2142. doi: 10.1007/s13300-023-01472-8. Epub 2023 Oct 6. Diabetes Ther. 2023. PMID: 37801224 Free PMC article. - Comparison of glucose-lowering agents after dual therapy failure in type 2 diabetes: A systematic review and network meta-analysis of randomized controlled trials.
Zaccardi F, Dhalwani NN, Dales J, Mani H, Khunti K, Davies MJ, Webb DR. Zaccardi F, et al. Diabetes Obes Metab. 2018 Apr;20(4):985-997. doi: 10.1111/dom.13185. Epub 2018 Jan 8. Diabetes Obes Metab. 2018. PMID: 29205774 - Efficacy and tolerability of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: A systematic review and network meta-analysis.
Hussein H, Zaccardi F, Khunti K, Davies MJ, Patsko E, Dhalwani NN, Kloecker DE, Ioannidou E, Gray LJ. Hussein H, et al. Diabetes Obes Metab. 2020 Jul;22(7):1035-1046. doi: 10.1111/dom.14008. Epub 2020 Mar 18. Diabetes Obes Metab. 2020. PMID: 32077218 Review. - Long-acting GLP-1RAs: An overview of efficacy, safety, and their role in type 2 diabetes management.
Chun JH, Butts A. Chun JH, et al. JAAPA. 2020 Aug;33(8):3-18. doi: 10.1097/01.JAA.0000669456.13763.bd. JAAPA. 2020. PMID: 32740121 Review.
Cited by
- Generalizability and treatment with sodium-glucose co-trasporter-2 inhibitors (SGLT2i) among patients with type 2 diabetes: an assessment using an Italian primary care database.
Antonazzo IC, Rozza D, Cortesi PA, Fornari C, Zanzottera Ferrari E, Paris C, Eteve-Pitsaer C, Gnesi M, Mele S, D'Amelio M, Maurizi AR, Palladino P, Mantovani LG, Mazzaglia G. Antonazzo IC, et al. Acta Diabetol. 2024 Aug 29. doi: 10.1007/s00592-024-02359-1. Online ahead of print. Acta Diabetol. 2024. PMID: 39207490 - GLP-1 receptor agonists' impact on cardio-renal outcomes and mortality in T2D with acute kidney disease.
Pan HC, Chen JY, Chen HY, Yeh FY, Sun CY, Huang TT, Wu VC. Pan HC, et al. Nat Commun. 2024 Jul 13;15(1):5912. doi: 10.1038/s41467-024-50199-y. Nat Commun. 2024. PMID: 39003287 Free PMC article. - Real-World Evaluation of GLP-1 Receptor Agonist Therapy Persistence, Adherence and Therapeutic Inertia Among Obese Adults with Type 2 Diabetes.
Palanca A, Ampudia-Blasco FJ, Calderón JM, Sauri I, Martinez-Hervás S, Trillo JL, Redón J, Real JT. Palanca A, et al. Diabetes Ther. 2023 Apr;14(4):723-736. doi: 10.1007/s13300-023-01382-9. Epub 2023 Feb 27. Diabetes Ther. 2023. PMID: 36847952 Free PMC article. - GLP-1 RAs in Spain: A Short Narrative Review of Their Use in Real Clinical Practice.
Romera I, Rubio-de Santos M, Artola S, Suárez Fernández C, Conget I. Romera I, et al. Adv Ther. 2023 Apr;40(4):1418-1429. doi: 10.1007/s12325-023-02442-z. Epub 2023 Feb 23. Adv Ther. 2023. PMID: 36821026 Free PMC article. Review. - Management of type 2 diabetes with a treat-to-benefit approach improved long-term cardiovascular outcomes under routine care.
Morieri ML, Longato E, Di Camillo B, Sparacino G, Avogaro A, Fadini GP. Morieri ML, et al. Cardiovasc Diabetol. 2022 Dec 9;21(1):274. doi: 10.1186/s12933-022-01712-4. Cardiovasc Diabetol. 2022. PMID: 36494815 Free PMC article.
References
- Heuvelman VD, Van Raalte DH, Smits MM. Cardiovascular effects of glucagon-like peptide 1 receptor agonists: from mechanistic studies in humans to clinical outcomes. Cardiovasc Res. 2020;116(5):916–930. - PubMed
Publication types
LinkOut - more resources
Full Text Sources