New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study - PubMed (original) (raw)
New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study
Ahmed Gaber et al. Molecules. 2021.
Abstract
Herein, we report the synthesis of eight new mononuclear and binuclear Co2+, Ni2+, Cu2+, and Zn2+ methoxy thiosemicarbazone (MTSC) complexes aiming at obtaining thiosemicarbazone complex with potent biological activity. The structure of the MTSC ligand and its metal complexes was fully characterized by elemental analysis, spectroscopic techniques (NMR, FTIR, UV-Vis), molar conductivity, thermogravimetric analysis (TG), and thermal differential analysis (DrTGA). The spectral and analytical data revealed that the obtained thiosemicarbazone-metal complexes have octahedral geometry around the metal center, except for the Zn2+-thiosemicarbazone complexes, which showed a tetrahedral geometry. The antibacterial and antifungal activities of the MTSC ligand and its (Co2+, Ni2+, Cu2+, and Zn2+) metal complexes were also investigated. Interestingly, the antibacterial activity of MTSC- metal complexes against examined bacteria was higher than that of the MTSC alone, which indicates that metal complexation improved the antibacterial activity of the parent ligand. Among different metal complexes, the MTSC- mono- and binuclear Cu2+ complexes showed significant antibacterial activity against Bacillus subtilis and Proteus vulgaris, better than that of the standard gentamycin drug. The in silico molecular docking study has revealed that the MTSC ligand could be a potential inhibitor for the oxidoreductase protein.
Keywords: antimicrobial; metal complexes; metallodrugs; methoxy thiosemicarbazone; molecular docking; transition metals.
Conflict of interest statement
The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures
Scheme 1
Structures of thiosemicarbazone in thione-thiol tautomerism.
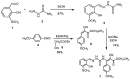
Figure 1
Reagents and conditions for the synthesis of the methoxy thiosemicarbazone (MTSC) ligand (7).
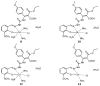
Figure 2
Proposed structures of 1:1 MTSC complexes (8, 10, 12, 14).
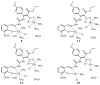
Figure 3
Proposed structures of 1:2 MTSC complexes (9, 11, 13, 15).
Figure 4
The 3D molecular docking of MTSC ligand against 3hb5-oxidoreductase protein (A) and speckle-type POZ protein (SPOP) protein binding (B).
Similar articles
- Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semicarbazone ligands.
Kasuga NC, Sekino K, Ishikawa M, Honda A, Yokoyama M, Nakano S, Shimada N, Koumo C, Nomiya K. Kasuga NC, et al. J Inorg Biochem. 2003 Aug 1;96(2-3):298-310. doi: 10.1016/s0162-0134(03)00156-9. J Inorg Biochem. 2003. PMID: 12888265 - Synthesis, characterization, thermal properties, antimicrobial evaluation, ADMET study, and molecular docking simulation of new mono Cu (II) and Zn (II) complexes with 2-oxoindole derivatives.
Ragab A, Ammar YA, Ezzat A, Mahmoud AM, Mohamed MBI, El-Tabl AS, Farag RS. Ragab A, et al. Comput Biol Med. 2022 Jun;145:105473. doi: 10.1016/j.compbiomed.2022.105473. Epub 2022 Apr 4. Comput Biol Med. 2022. PMID: 35395516 - Spectroscopic and biological approach of Ni(II), Cu(II) and Co(II) complexes of 4-methoxy/ethoxybenzaldehyde thiosemicarbazone glyoxime.
Babahan I, Eyduran F, Coban EP, Orhan N, Kazar D, Biyik H. Babahan I, et al. Spectrochim Acta A Mol Biomol Spectrosc. 2014;121:205-15. doi: 10.1016/j.saa.2013.10.040. Epub 2013 Oct 25. Spectrochim Acta A Mol Biomol Spectrosc. 2014. PMID: 24239764 - Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)-A Review.
Aly AA, Abdallah EM, Ahmed SA, Rabee MM, Bräse S. Aly AA, et al. Molecules. 2023 Feb 14;28(4):1808. doi: 10.3390/molecules28041808. Molecules. 2023. PMID: 36838796 Free PMC article. Review. - Recent progress in thiocarbazone metal complexes for cancer therapy via mitochondrial signalling pathway.
Zheng Y, An H, Qi J, Li J. Zheng Y, et al. Front Chem. 2024 May 30;12:1424022. doi: 10.3389/fchem.2024.1424022. eCollection 2024. Front Chem. 2024. PMID: 38873408 Free PMC article. Review.
Cited by
- Effect of Salt Stress and Foliar Application of Salicylic Acid on Morphological, Biochemical, Anatomical, and Productivity Characteristics of Cowpea (Vigna unguiculata L.) Plants.
El-Taher AM, Abd El-Raouf HS, Osman NA, Azoz SN, Omar MA, Elkelish A, Abd El-Hady MAM. El-Taher AM, et al. Plants (Basel). 2021 Dec 31;11(1):115. doi: 10.3390/plants11010115. Plants (Basel). 2021. PMID: 35009118 Free PMC article. - Exploring the antibacterial potential of plant extracts and essential oils against Bacillus thermophilus in beet sugar for enhanced sucrose retention: a comparative assessment and implications.
Yousef MM, Zohri AA, Darwish AMG, Shamseldin A, Kabeil SA, Abdelkhalek A, Binsuwaidan R, Jaremko M, Alshwyeh HA, Hafez EE, Saied EM. Yousef MM, et al. Front Microbiol. 2023 Jul 20;14:1219823. doi: 10.3389/fmicb.2023.1219823. eCollection 2023. Front Microbiol. 2023. PMID: 37547698 Free PMC article. - Insight into the phytochemical profile and antimicrobial activities of Amomum subulatum and Amomum xanthioides: an in vitro and in silico study.
Alruhaili MH, Almuhayawi MS, Gattan HS, Alharbi MT, Nagshabandi MK, Jaouni SKA, Selim S, AbdElgawad H. Alruhaili MH, et al. Front Plant Sci. 2023 Apr 20;14:1136961. doi: 10.3389/fpls.2023.1136961. eCollection 2023. Front Plant Sci. 2023. PMID: 37152127 Free PMC article. - Enhancing Dissolution Rate and Antibacterial Efficiency of Azithromycin through Drug-Drug Cocrystals with Paracetamol.
Ul Islam N, Khan E, Naveed Umar M, Shah A, Zahoor M, Ullah R, Bari A. Ul Islam N, et al. Antibiotics (Basel). 2021 Aug 4;10(8):939. doi: 10.3390/antibiotics10080939. Antibiotics (Basel). 2021. PMID: 34438989 Free PMC article. - Secondary Metabolites of Actinomycetales as Potent Quorum Sensing Inhibitors Targeting Gram-Positive Pathogens: In Vitro and In Silico Study.
Desouky SE, Abu-Elghait M, Fayed EA, Selim S, Yousuf B, Igarashi Y, Abdel-Wahab BA, Mohammed Alsuhaibani A, Sonomoto K, Nakayama J. Desouky SE, et al. Metabolites. 2022 Mar 15;12(3):246. doi: 10.3390/metabo12030246. Metabolites. 2022. PMID: 35323689 Free PMC article.
References
- Balabanova Y., Gilsdorf A., Buda S., Burger R., Eckmanns T., Gaertner B., Gross U., Haas W., Hamouda O., Huebner J., et al. Communicable Diseases Prioritized for Surveillance and Epidemiological Research: Results of a Standardized Prioritization Procedure in Germany, 2011. PLoS ONE. 2011;6:e25691. doi: 10.1371/journal.pone.0025691. - DOI - PMC - PubMed
- Quiroga A.G., Ranninger C.N. Contribution to the SAR field of metallated and coordination complexes: Studies of the palladium and platinum derivatives with selected thiosemicarbazones as antitumoral drugs. Coord. Chem. Rev. 2004;248:119–133. doi: 10.1016/j.cct.2003.11.004. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous