The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex - PubMed (original) (raw)
The Schizosaccharomyces pombe spindle checkpoint protein mad2p blocks anaphase and genetically interacts with the anaphase-promoting complex
X He et al. Proc Natl Acad Sci U S A. 1997.
Abstract
The spindle checkpoint monitors mitotic spindle integrity and the attachment of kinetochores to the spindle. Upon sensing a defect the checkpoint blocks cell cycle progression and thereby prevents chromosome missegregation. Previous studies in budding yeast show that the activated spindle checkpoint inhibits the onset of anaphase by an unknown mechanism. One possible target of the spindle checkpoint is anaphase promoting complex (APC), which controls all postmetaphase events that are blocked by spindle checkpoint activation. We have isolated mad2, a spindle checkpoint component in fission yeast, and shown that mad2 overexpression activates the checkpoint and causes a cell cycle arrest at the metaphase-to-anaphase transition. In addition to the observation that mad2-induced arrest can be partially relieved by mitosis-promoting factor inactivation, we present genetic evidence consistent with the hypothesis that the spindle checkpoint imposes a cell cycle arrest by inhibiting APC-dependent proteolysis.
Figures
Figure 1
S. pombe mad2p functions as a spindle-assembly checkpoint protein. (A) Open reading frame of S. pombe mad2 (GenBank accession no. U72150) aligned with the S. cerevisiae Mad2p (GenBank accession no. U14132). Identical amino acids are labeled with stars and conserved amino acids are labeled with dots. (B) Hypersensitivity of _mad2_Δ to thiabendazole (TBZ) treatment. Serial dilutions (1/5) of an equal number of _mad2_Δ and wild-type cells were spotted on Edinburgh minimum medium plates with the indicated concentration of TBZ. (C) Viability test of the single mutants _mad2_Δ and nda3–311cs and the double mutant _mad2_Δ nda3–311cs after a transient incubation at 18°C. Colonies on each plate were counted and the percentage of viable cells was calculated by normalization to the colony number of the 0-hr samples. (D) Flow-cytometric analysis of DNA content of the single mutants _mad2_Δ and nda3–311cs and the double mutant _mad2_Δ nda3–311cs after a transient incubation at 20°C. The relative fluorescence corresponding to 2C and 4C DNA content is marked by arrows; hours indicate the length of 20°C incubation.
Figure 2
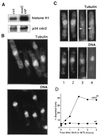
mad2 overexpression arrests cells at the metaphase-to-anaphase transition, and subsequent cdc2ts inactivation promotes septum formation. (A) MPF H1 kinase assay. MPF protein complex was immunoprecipitated from total protein extract by anti-cdc2 antiserum and subjected to H1 kinase assay. (Upper) Autoradiography of [γ-32P]ATP-labeled histone H1. (Lower) Western blot of immunoprecipitated p34 cdc2. (B Upper) Wild-type cells overexpressing mad2 in which microtubules were visualized with anti-tubulin indirect immunofluorescence. (Lower) The same cells stained with DAPI to visualize DNA. (C) cdc2–33ts and wild-type cells, arrested by mad2 overexpression at 25°C, were shifted to 36°C to inactivate MPF. Tubulin (Upper) and DNA (Lower) were visualized as in B. Arrows in cells 3 and 4 (Upper) indicate cytoplasmic microtubules. (D) cdc2–33ts and wild-type cells, arrested by mad2 overexpression at 25°C, were shifted to 36°C to inactivate MPF. Cells were fixed with ethanol, and the percentage of septated cells was counted using light microscopy.
Figure 3
APC mutants are hypersensitive to mad2 overexpression. Wild-type cells and APC subunit mutants (cut9–665ts and nuc2–663ts) were transformed with pREP3X vector and a set of mad2 overexpression constructs: pREP3X-mad2, pREP41X-mad2, and pREP81X-mad2. Transformants were streaked on EMM plates in the absence of thiamine so that three levels of mad2 overexpression were achieved via the three different strength nmt1 promoters: pREP3X-mad2, high; pREP41X-mad2, medium; and pREP81X-mad2, low (27). On each plate, wild-type transformants were streaked on the left and the mutant transformants on the right. The plates were incubated at 25°C.
Figure 4
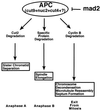
A model of the mechanism by which mad2 overexpression blocks the metaphase-to-anaphase transition. APC targets specific proteins for degradation, which is required for three different postmetaphase events. Cyclin B degradation is required for the exit from mitosis. Cut2p degradation is required for sister chromosome separation. The protein whose degradation is required for spindle elongation has not yet been identified. mad2 overexpression mimics activation of the spindle checkpoint and blocks the metaphase-to-anaphase transition by directly or indirectly inhibiting APC.
Similar articles
- Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint.
Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Kim SH, et al. Science. 1998 Feb 13;279(5353):1045-7. doi: 10.1126/science.279.5353.1045. Science. 1998. PMID: 9461438 - Fission yeast Mad3p is required for Mad2p to inhibit the anaphase-promoting complex and localizes to kinetochores in a Bub1p-, Bub3p-, and Mph1p-dependent manner.
Millband DN, Hardwick KG. Millband DN, et al. Mol Cell Biol. 2002 Apr;22(8):2728-42. doi: 10.1128/MCB.22.8.2728-2742.2002. Mol Cell Biol. 2002. PMID: 11909965 Free PMC article. - Mitotic arrest: Mad2 prevents sleepy from waking up the APC.
Elledge SJ. Elledge SJ. Science. 1998 Feb 13;279(5353):999-1000. doi: 10.1126/science.279.5353.999. Science. 1998. PMID: 9490488 No abstract available. - Regulation of APC-Cdc20 by the spindle checkpoint.
Yu H. Yu H. Curr Opin Cell Biol. 2002 Dec;14(6):706-14. doi: 10.1016/s0955-0674(02)00382-4. Curr Opin Cell Biol. 2002. PMID: 12473343 Review. - Control of metaphase-anaphase progression by proteolysis: cyclosome function regulated by the protein kinase A pathway, ubiquitination and localization.
Yanagida M, Yamashita YM, Tatebe H, Ishii K, Kumada K, Nakaseko Y. Yanagida M, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Sep 29;354(1389):1559-69; discussion 1569-70. doi: 10.1098/rstb.1999.0499. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10582241 Free PMC article. Review.
Cited by
- Sim4: a novel fission yeast kinetochore protein required for centromeric silencing and chromosome segregation.
Pidoux AL, Richardson W, Allshire RC. Pidoux AL, et al. J Cell Biol. 2003 Apr 28;161(2):295-307. doi: 10.1083/jcb.200212110. J Cell Biol. 2003. PMID: 12719471 Free PMC article. - ago1 and dcr1, two core components of the RNA interference pathway, functionally diverge from rdp1 in regulating cell cycle events in Schizosaccharomyces pombe.
Carmichael JB, Provost P, Ekwall K, Hobman TC. Carmichael JB, et al. Mol Biol Cell. 2004 Mar;15(3):1425-35. doi: 10.1091/mbc.e03-06-0433. Epub 2003 Dec 29. Mol Biol Cell. 2004. PMID: 14699070 Free PMC article. - Uncoupling of Mitosis and Cytokinesis Upon a Prolonged Arrest in Metaphase Is Influenced by Protein Phosphatases and Mitotic Transcription in Fission Yeast.
Chica N, Portantier M, Nyquist-Andersen M, Espada-Burriel S, Lopez-Aviles S. Chica N, et al. Front Cell Dev Biol. 2022 Jul 18;10:876810. doi: 10.3389/fcell.2022.876810. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35923846 Free PMC article. - Phosphorylation state defines discrete roles for monopolin in chromosome attachment and spindle elongation.
Choi SH, Péli-Gulli MP, Mcleod I, Sarkeshik A, Yates JR 3rd, Simanis V, McCollum D. Choi SH, et al. Curr Biol. 2009 Jun 23;19(12):985-95. doi: 10.1016/j.cub.2009.05.042. Epub 2009 Jun 11. Curr Biol. 2009. PMID: 19523829 Free PMC article.
References
- Harwell L H, Weinert T A. Science. 1989;266:629–634. - PubMed
- Paulovich A G, Toczyski D P, Hartwell L H. Cell. 1997;88:315–321. - PubMed
- Rudner A D, Murray A W. Curr Opin Cell Biol. 1996;8:773–780. - PubMed
- Wells W A E. Trends Cell Biol. 1996;6:228–234. - PubMed
- Chen R, Waters J C, Salmon E D, Murray A W. Science. 1996;274:242–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases