E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE - PubMed (original) (raw)
E1B 19K inhibits Fas-mediated apoptosis through FADD-dependent sequestration of FLICE
D Perez et al. J Cell Biol. 1998.
Abstract
E1B 19K, the adenovirus Bcl-2 homologue, is a potent inhibitor of apoptosis induced by various stimuli including Fas and tumor necrosis factor-alpha. Fas and TNFR-1 belong to a family of cytokine-activated receptors that share key components in their signaling pathways, Fas-associating protein with death domain (FADD) and FADD-like interleukin-1beta-converting enzyme (FLICE), to induce an apoptotic response. We demonstrate here that E1B 19K and Bcl-xL are able to inhibit apoptosis induced by FADD, but not FLICE. Surprisingly, apoptosis was abrogated by E1B 19K and Bcl-xL when FADD and FLICE were coexpressed. Immunofluorescence studies demonstrated that FADD expression produced large insoluble death effector filaments that may represent oligomerized FADD. E1B 19K expression disrupted FADD filament formation causing FADD and FLICE to relocalize to membrane and cytoskeletal structures where E1B 19K is normally localized. E1B 19K, however, does not detectably bind to FADD, nor does it inhibit FADD and FLICE from being recruited to the death-inducing signaling complex (DISC) when Fas is stimulated. Thus, E1B 19K may inhibit Fas-mediated cell death downstream of FADD recruitment of FLICE but upstream of FLICE activation by disrupting FADD oligomerization and sequestering an essential component of the DISC.
Figures
Figure 8
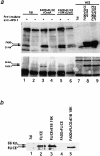
(a) FADD and FLICE coimmunoprecipitate with Fas in the presence of E1B 19K. HeLa cells were transfected with 7dl vector control (lanes 1 and 2), FADD, FLICE, and CrmA (lanes 3 and 4), or FADD, FLICE, E1B 19K, and CrmA (lanes 5 and 6) and then immunoprecipitated with an anti–APO-1 antibody 48 h posttransfection. In lanes 1, 3, and 5, cells were first stimulated with anti–APO-1 antibody, lysed, and then immunoprecipitated with the addition of protein A–Sepharose beads. In lanes 2, 4, and 6, cells were lysed first and then immunoprecipitated with anti–APO-1 antibody. Lanes 7–9 correspond to whole cell extracts used to verify equal protein expression. Samples were resolved on a 15% SDS-PAGE and subjected to Western analysis with an anti-FADD polyclonal antibody (top panel) and an anti-HA polyclonal antibody (bottom panel). (b) E1B 19K blocks upstream of FLICE activation. HeLa cells were transfected with 7dl vector control, FLICE, or FLICE plus FADD alone or in the presence of E1B 19K at concentrations indicated in the viability assay (refer to Materials and Methods). Whole cell extracts were made 48 h posttransfection, resolved by SDS-PAGE, and then immunoblotted with an anti-HA polyclonal antibody.
Figure 1
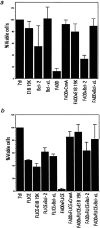
(a) E1B 19K and Bcl-xL abrogate FADD-mediated apoptosis. HeLa cells were transfected with FADD alone or cotransfected with Bcl-2, Bcl-xL, or E1B 19K. Bcl-2, Bcl-xL, and E1B 19K were also each expressed individually as controls. A β-gal viability assay was performed 48 h posttransfection. The percentage of viable cells was compared with 7dl transfected vector control cells. The values represent data from a single experiment that was reproducible in three independent experiments. (b) E1B 19K and Bcl-xL block FLICE-mediated cell death only in the presence of the FADD adaptor molecule. HeLa cells were transfected with FLICE alone or coexpressed with Bcl-2, Bcl-xL, E1B 19K, or FADD. Cells were transfected with FADD and FLICE in the presence of Bcl-2, Bcl-xL, or E1B 19K. Bcl-2, Bcl-xL, and E1B 19K were also each expressed alone as a control. Viability was assessed 48 h posttransfection and compared with the 7dl vector control.
Figure 2
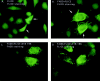
FLICE has a diffuse cytosolic and nuclear staining pattern and E1B 19K does not alter its subcellular localization. HeLa cells were transfected with the expression plasmids indicated and processed for indirect immunofluorescence 48 h posttransfection. HeLa cells were transfected with the 7dl vector control and stained with an anti-HA polyclonal antibody (A). HeLa cells were transfected with HA-tagged FLICE and stained with an anti-HA polyclonal antibody (B). In C and D, cells were transfected with HA-tagged FLICE plus E1B 19K and stained with an anti-HA polyclonal antibody (C) and an antibody directed against E1B 19K (D). Cells were also transfected with CrmA to retain cell viability.
Figure 2
FLICE has a diffuse cytosolic and nuclear staining pattern and E1B 19K does not alter its subcellular localization. HeLa cells were transfected with the expression plasmids indicated and processed for indirect immunofluorescence 48 h posttransfection. HeLa cells were transfected with the 7dl vector control and stained with an anti-HA polyclonal antibody (A). HeLa cells were transfected with HA-tagged FLICE and stained with an anti-HA polyclonal antibody (B). In C and D, cells were transfected with HA-tagged FLICE plus E1B 19K and stained with an anti-HA polyclonal antibody (C) and an antibody directed against E1B 19K (D). Cells were also transfected with CrmA to retain cell viability.
Figure 3
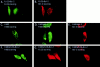
Overexpression of FADD and not a FADD-DN mutant forms filamentous structures that are disrupted by E1B 19K expression. HeLa cells were transfected with the 7dl vector control and stained with an anti-FADD antibody (A). In B, cells were transfected with AU1-tagged FADD and protein expression detected with an anti-FADD polyclonal antibody. In C and D, HeLa cells coexpressed AU1-tagged FADD and E1B 19K and were stained with an anti-FADD polyclonal antibody (C) and an anti-E1B 19K monoclonal antibody (D). HeLa cells were transfected with 7dl vector control and stained with an anti-AU1 monoclonal antibody (E). Cells were transfected with AU1-tagged FADD-DN alone (F) or in the presence of E1B 19K (G and H). FADD-DN protein expression was visualized with an anti-AU1 monoclonal antibody (F and G) and E1B 19K detected with a polyclonal antibody directed against it (H). All cells were also transfected with CrmA to retain cell viability.
Figure 4
E1B 19K disrupts FADD filament formation and colocalizes with FLICE in the presence of the FADD adaptor molecule. HeLa cells were transfected with HA-tagged FLICE or coexpressed with HA-tagged FLICE plus AU1-tagged FADD (A and B, respectively). FLICE was visualized with an anti-HA antibody (A and B). In C–E HeLa cells transiently coexpressed AU1-tagged FADD, HA-tagged FLICE, and E1B 19K. A polyclonal antibody directed against FADD (C), an anti-HA polyclonal antibody directed against the epitope tag on FLICE (D), and monoclonal antibody directed against E1B 19K (E) was used to visualize protein expression. All cells were also transfected with CrmA to retain cell viability.
Figure 4
E1B 19K disrupts FADD filament formation and colocalizes with FLICE in the presence of the FADD adaptor molecule. HeLa cells were transfected with HA-tagged FLICE or coexpressed with HA-tagged FLICE plus AU1-tagged FADD (A and B, respectively). FLICE was visualized with an anti-HA antibody (A and B). In C–E HeLa cells transiently coexpressed AU1-tagged FADD, HA-tagged FLICE, and E1B 19K. A polyclonal antibody directed against FADD (C), an anti-HA polyclonal antibody directed against the epitope tag on FLICE (D), and monoclonal antibody directed against E1B 19K (E) was used to visualize protein expression. All cells were also transfected with CrmA to retain cell viability.
Figure 5
Bcl-2 does not substantially disrupt FADD filaments. HeLa cells were transfected with the expression plasmids indicated and indirect immunofluorescence was performed 48 h posttransfection. HeLa cells were cotransfected with HA-tagged FLICE and Myc-tagged Bcl-2 and then stained with an anti-HA polyclonal antibody (A) or an anti-Myc monoclonal antibody (B). In C, cells were transfected with AU1-tagged FADD alone and visualized with an anti-FADD polyclonal antibody. In D and E, cells were transfected with AU1-tagged FADD and Myc-tagged Bcl-2 and protein expression detected with an anti-FADD polyclonal antibody (D) and an anti-Myc monoclonal antibody (E). In panels F–H, cells were transfected with AU1-tagged FADD, HA-tagged FLICE, and Myc-tagged Bcl-2. Proteins were visualized with anti-FADD polyclonal antibody (F), anti-HA monoclonal antibody (G), and anti-Myc monoclonal antibody (H). All cells were also transfected with CrmA to retain cell viability.
Figure 6
Bcl-xL attenuates the formation of FADD filaments. HeLa cells were transfected with the expression plasmids indicated and indirect immunofluorescence was performed 48 h posttransfection. HeLa cells were cotransfected with HA-tagged FLICE and Flag-tagged Bcl-xL and then stained with an anti-HA polyclonal antibody (A) or an anti-Flag monoclonal antibody (B). Cells were transfected with AU1-tagged FADD alone and visualized with an anti-FADD polyclonal antibody (C). In D and E, cells were transfected with AU1-tagged FADD and Flag-tagged Bcl-xL and protein expression was detected with an anti-FADD polyclonal antibody (D) and an anti-Flag monoclonal antibody (E). In panels F–H, cells were transfected with AU1-tagged FADD, HA-tagged FLICE, and Flag-tagged Bcl-xL. Proteins were visualized with anti-FADD polyclonal antibody (F), anti-HA monoclonal antibody (G), and anti-Flag monoclonal antibody (H). All cells were also transfected with CrmA to retain cell viability.
Figure 7
E1B 19K does not associate with FADD or FLICE in vitro. In vitro translated [35S]methionine FADD, FLICE, FADD-DN, E1B 19K, and Nbk/Bik were incubated as indicated and immunoprecipitated with an anti-FADD antibody (lanes 1 and 2) and an anti-E1B 19K antibody (lanes 3–6). Arrows, in vitro translated products.
Similar articles
- E1B 19,000-molecular-weight protein interacts with and inhibits CED-4-dependent, FLICE-mediated apoptosis.
Han J, Wallen HD, Nuñez G, White E. Han J, et al. Mol Cell Biol. 1998 Oct;18(10):6052-62. doi: 10.1128/MCB.18.10.6052. Mol Cell Biol. 1998. PMID: 9742122 Free PMC article. - Tumor necrosis factor-alpha induces Bax-Bak interaction and apoptosis, which is inhibited by adenovirus E1B 19K.
Sundararajan R, Cuconati A, Nelson D, White E. Sundararajan R, et al. J Biol Chem. 2001 Nov 30;276(48):45120-7. doi: 10.1074/jbc.M106386200. Epub 2001 Sep 24. J Biol Chem. 2001. PMID: 11571294 - A novel family of viral death effector domain-containing molecules that inhibit both CD-95- and tumor necrosis factor receptor-1-induced apoptosis.
Hu S, Vincenz C, Buller M, Dixit VM. Hu S, et al. J Biol Chem. 1997 Apr 11;272(15):9621-4. doi: 10.1074/jbc.272.15.9621. J Biol Chem. 1997. PMID: 9092488 - Apoptosis induced by a chimeric Fas/FLICE receptor: lack of requirement for Fas- or FADD-binding proteins.
Memon SA, Hou J, Moreno MB, Zacharchuk CM. Memon SA, et al. J Immunol. 1998 Mar 1;160(5):2046-9. J Immunol. 1998. PMID: 9498739
Cited by
- A Dual Role for FADD in Human Precursor T-Cell Neoplasms.
Marín-Rubio JL, Vela-Martín L, Gudgeon J, Pérez-Gómez E, Sidgwick FR, Trost M, Cunningham DL, Santos J, Fernández-Piqueras J, Villa-Morales M. Marín-Rubio JL, et al. Int J Mol Sci. 2022 Dec 2;23(23):15157. doi: 10.3390/ijms232315157. Int J Mol Sci. 2022. PMID: 36499482 Free PMC article. - A motif within the armadillo repeat of Parkinson's-linked LRRK2 interacts with FADD to hijack the extrinsic death pathway.
Antoniou N, Vlachakis D, Memou A, Leandrou E, Valkimadi PE, Melachroinou K, Re DB, Przedborski S, Dauer WT, Stefanis L, Rideout HJ. Antoniou N, et al. Sci Rep. 2018 Feb 22;8(1):3455. doi: 10.1038/s41598-018-21931-8. Sci Rep. 2018. PMID: 29472595 Free PMC article. - Prevention of apoptosis by the interaction between FIH1 and Bax.
Yan B, Kong M, Chen YH. Yan B, et al. Mol Cell Biochem. 2011 Feb;348(1-2):1-9. doi: 10.1007/s11010-010-0631-2. Epub 2010 Nov 11. Mol Cell Biochem. 2011. PMID: 21069436 - Oncolytic Viruses for Cancer Therapy: Overcoming the Obstacles.
Wong HH, Lemoine NR, Wang Y. Wong HH, et al. Viruses. 2010 Jan;2(1):78-106. doi: 10.3390/v2010078. Viruses. 2010. PMID: 20543907 Free PMC article. - Role of the polarity determinant crumbs in suppressing mammalian epithelial tumor progression.
Karp CM, Tan TT, Mathew R, Nelson D, Mukherjee C, Degenhardt K, Karantza-Wadsworth V, White E. Karp CM, et al. Cancer Res. 2008 Jun 1;68(11):4105-15. doi: 10.1158/0008-5472.CAN-07-6814. Cancer Res. 2008. PMID: 18519669 Free PMC article.
References
- Armstrong RC, Aja T, Xioang J, Gaur S, Krebs JF, Hoang K, Bai X, Korsmeyer SJ, Karanewsky DS, Fritz LC, Tomaselli KJ. Fas- induced activation of the cell death-related protease CPP32 is inhibited by Bcl-2 and by ICE family protease inhibitors. J Biol Chem. 1996;27128:16850–16855. - PubMed
- Beidler DR, Tewari M, Friesen PD, Poirier G, Dixit VM. The baculovirus p35 protein inhibits Fas- and tumor necrosis factor-induced apoptosis. J Biol Chem. 1995;270:16526–16528. - PubMed
- Bellgrau D, Gold D, Selawry H, Moore J, Franzusoff A, Duke RC. A role for CD95 ligand in preventing graft rejection. Nature. 1995;377:630–632. - PubMed
- Boldin MP, Varfolomeev EE, Pancer Z, Mett IL, Camonis JH, Wallach D. A novel protein that interacts with the death domain of Fas/ APO1 contains a sequence motif related to the death domain. J Biol Chem. 1995;270:7795–7798. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous