Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant - PubMed (original) (raw)
Eskimo1 mutants of Arabidopsis are constitutively freezing-tolerant
Z Xin et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Temperate plants develop a greater ability to withstand freezing in response to a period of low but nonfreezing temperatures through a complex, adaptive process of cold acclimation. Very little is known about the signaling processes by which plants perceive the low temperature stimulus and transduce it into the nucleus to activate genes needed for increased freezing tolerance. To help understand the signaling processes, we have isolated mutants of Arabidopsis that are constitutively freezing-tolerant in the absence of cold acclimation. Freezing tolerance of wild-type Arabidopsis was increased from -5.5 degreesC to -12.6 degreesC by cold acclimation whereas the freezing tolerance of 26 mutant lines ranged from -6.8 degreesC to -10.6 degreesC in the absence of acclimation. Plants with mutations at the eskimo1 (esk1) locus accumulated high levels of proline, a compatible osmolyte, but did not exhibit constitutively increased expression of several cold-regulated genes involved in freezing tolerance. RNA gel blot analysis suggested that proline accumulation in esk1 plants was mediated by regulation of transcript levels of genes involved in proline synthesis and degradation. The characterization of esk1 mutants and results from other mutants suggest that distinct signaling pathways activate different aspects of cold acclimation and that activation of one pathway can result in considerable freezing tolerance without activation of other pathways.
Figures
Figure 1
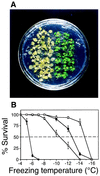
Freezing survival of wild-type and esk1 plants. (A) Nonacclimated wild-type (Left) and esk1 plants were frozen at −8°C under the protocol described under Methods. (B) Percent survival of nonacclimated and acclimated plants after freezing to different temperatures. Nonacclimated wild-type (•) and esk1 (○) were frozen in a temperature-controlled chamber as described under Methods. At the temperatures shown, samples of plants were removed from the chamber, allowed to recover, and scored for survival. Alternatively, wild-type (▪) and esk1 (□) plants were cold acclimated at 4°C for 2 days before being subjected to the same freezing test. The data are means ± SE for three separate experiments.
Figure 2
Growth habits of wild-type (Left) and esk1 plants. (A) Plants grown for 30 days at 22°C under 150 μmol quanta m−2⋅s−1 continuous light. (B) Plants grown for 7 days at 22°C and then for 60 days at 4°C under 90 μmol quanta m−2⋅s−1 continuous light.
Figure 3
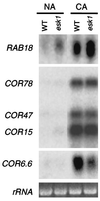
Gel-blot analysis of transcripts from five cold-regulated genes in wild-type (WT) and esk1 Arabidopsis. Plants were grown at 22°C under 150 μmol quanta m−2⋅s−1. Total RNA (10 μg) isolated from leaf tissue of nonacclimated plants (NA) or from plants cold acclimated at 4°C for 2 days (CA) was separated on a 1.2% agarose-formaldehyde gel and probed successively with cDNAs corresponding to the cold-regulated genes indicated. The ribosomal 25S rRNA was visualized on the nylon membrane by using ethidium bromide to demonstrate equal loading. Quantitative comparisons reported in the text are based on PhosphorImager analyses of the blots. The experiment was repeated four times with similar results.
Figure 4
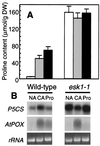
Proline accumulation and expression of proline metabolism genes in wild-type (WT) and esk1 Arabidopsis. (A) Proline levels in nonacclimated plants (open bars), in plants cold acclimated at 4°C for 2 days (shaded bars), and in plants 10 hr after application of 100 mM proline (solid bars). Harvested leaf tissue was frozen in liquid nitrogen and lyophilized. Free amino acids were extracted and quantified by using an amino acid analyzer. (B) Gel-blot analysis of transcript levels for genes encoding Δ1-pyrroline-5-carboxylate synthetase (P5CS) and proline oxidase (AtPOX). Methods are those described in Fig. 3.
Similar articles
- ESKIMO1 is a key gene involved in water economy as well as cold acclimation and salt tolerance.
Bouchabke-Coussa O, Quashie ML, Seoane-Redondo J, Fortabat MN, Gery C, Yu A, Linderme D, Trouverie J, Granier F, Téoulé E, Durand-Tardif M. Bouchabke-Coussa O, et al. BMC Plant Biol. 2008 Dec 7;8:125. doi: 10.1186/1471-2229-8-125. BMC Plant Biol. 2008. PMID: 19061521 Free PMC article. - Mutational Evidence for the Critical Role of CBF Transcription Factors in Cold Acclimation in Arabidopsis.
Zhao C, Zhang Z, Xie S, Si T, Li Y, Zhu JK. Zhao C, et al. Plant Physiol. 2016 Aug;171(4):2744-59. doi: 10.1104/pp.16.00533. Epub 2016 Jun 1. Plant Physiol. 2016. PMID: 27252305 Free PMC article. - Arabidopsis ESK1 encodes a novel regulator of freezing tolerance.
Xin Z, Mandaokar A, Chen J, Last RL, Browse J. Xin Z, et al. Plant J. 2007 Mar;49(5):786-99. doi: 10.1111/j.1365-313X.2006.02994.x. Plant J. 2007. PMID: 17316173 - Gene Regulatory Networks Mediating Cold Acclimation: The CBF Pathway.
Barrero-Gil J, Salinas J. Barrero-Gil J, et al. Adv Exp Med Biol. 2018;1081:3-22. doi: 10.1007/978-981-13-1244-1_1. Adv Exp Med Biol. 2018. PMID: 30288701 Review. - Natural Variation in Freezing Tolerance and Cold Acclimation Response in Arabidopsis thaliana and Related Species.
Zuther E, Lee YP, Erban A, Kopka J, Hincha DK. Zuther E, et al. Adv Exp Med Biol. 2018;1081:81-98. doi: 10.1007/978-981-13-1244-1_5. Adv Exp Med Biol. 2018. PMID: 30288705 Review.
Cited by
- Recent Insights into the Physio-Biochemical and Molecular Mechanisms of Low Temperature Stress in Tomato.
Lee K, Kang H. Lee K, et al. Plants (Basel). 2024 Sep 28;13(19):2715. doi: 10.3390/plants13192715. Plants (Basel). 2024. PMID: 39409585 Free PMC article. Review. - Plant Cell Wall Polysaccharide _O_-Acetyltransferases.
Zhong R, Zhou D, Chen L, Rose JP, Wang BC, Ye ZH. Zhong R, et al. Plants (Basel). 2024 Aug 19;13(16):2304. doi: 10.3390/plants13162304. Plants (Basel). 2024. PMID: 39204739 Free PMC article. Review. - Integrity of xylan backbone affects plant responses to drought.
Barbut FR, Cavel E, Donev EN, Gaboreanu I, Urbancsok J, Pandey G, Demailly H, Jiao D, Yassin Z, Derba-Maceluch M, Master ER, Scheepers G, Gutierrez L, Mellerowicz EJ. Barbut FR, et al. Front Plant Sci. 2024 Jun 25;15:1422701. doi: 10.3389/fpls.2024.1422701. eCollection 2024. Front Plant Sci. 2024. PMID: 38984158 Free PMC article. - Exploring cotton SFR2's conundrum in response to cold stress.
Surber SM, Thien Thao NP, Smith CN, Shomo ZD, Barnes AC, Roston RL. Surber SM, et al. Plant Signal Behav. 2024 Dec 31;19(1):2362518. doi: 10.1080/15592324.2024.2362518. Epub 2024 Jun 5. Plant Signal Behav. 2024. PMID: 38836385 Free PMC article. - Metabolic and transcriptomic profiling during wheat seed development under progressive drought conditions.
Mega R, Kim JS, Tanaka H, Ishii T, Abe F, Okamoto M. Mega R, et al. Sci Rep. 2023 Sep 11;13(1):15001. doi: 10.1038/s41598-023-42093-2. Sci Rep. 2023. PMID: 37696863 Free PMC article.
References
- Levitt J. Responses of Plants to Environmental Stresses. Orlando: Academic; 1980. , 497 pp.
- Boyer J S. Science. 1982;218:443–448. - PubMed
- Brown I M, Gaugler R. J Thermal Biol. 1996;21:115–121.
- Kostal V, Simek P. J Insect Physiol. 1996;42:727–733.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases