Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study - PubMed (original) (raw)
Entry of Listeria monocytogenes into neurons occurs by cell-to-cell spread: an in vitro study
S Dramsi et al. Infect Immun. 1998 Sep.
Abstract
Listeria monocytogenes is an intracellular pathogen that causes severe central nervous system infection in humans and animals. The ability of this bacterium to penetrate nerve cells was investigated by using rat spinal cell cultures. Entry into distinct cell types, i. e., glial cells and neurons, was monitored by a differential immunofluorescence technique with antibodies against cell type-specific markers and the bacterial pathogen. L. monocytogenes was detected predominantly within macrophages constituting the microglia. Astrocytes and oligodendrocytes, the major components of macroglia, were infected to a lesser extent. Surprisingly, Listeria innocua, a noninvasive and nonpathogenic species, also has the capacity to enter into these three types of glial cells. Entry into neurons was a very rare event. In contrast, we found that L. monocytogenes could efficiently invade neurons when these latter cells were cocultivated with Listeria-infected mouse macrophages. In this case, infection of neurons occurs by cell-to-cell spread via an actA-dependent mechanism. These data support the notion that infected phagocytes can be vectors by which L. monocytogenes gains access to privileged niches such as the central nervous system.
Figures
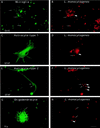
FIG. 1
Entry of L. monocytogenes into glial cell cultures. Glial cells were incubated with L. monocytogenes for 40 min, unbound bacteria were washed away, and the cells were resuspended in medium containing gentamicin and incubated for 2 h. The cells were fixed and stained with antibodies against cell type-specific markers (Ox42, GFAP, or Rip) and against the bacterial pathogen. The different types of glial cells are shown on the left panels. Differential immunofluorescence labeling of the bacteria was performed in order to discriminate between extracellular and intracellular bacteria, as previously described (see Material and Methods). Extracellular bacteria are indicated by thin arrows and are both green and red whereas intracellular bacteria, indicated by thick arrows, are only red. Two microglial cells are shown in panel A and a bulk of intracellular Listeria cells is shown in panel B. Astrocyte type 1, astrocyte type 2, and oligodendrocyte are shown in panels C, E, and G, respectively, and extracellular L. monocytogenes (thin arrows) and intracellular L. monocytogenes (thick arrows) are shown in panels D, F, and H. Scale bar, 10 μm.
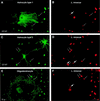
FIG. 2
Entry of L. innocua into glial cell cultures. Details of this infection experiment and immunofluorescence analysis are the same as those described in the legend for Fig. 1 except that L. innocua, a noninvasive and nonpathogenic species of the genus Listeria, was used.
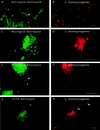
FIG. 3
L. monocytogenes can move and spread from cell to cell in glial cell cultures. Glial cells were incubated with L. monocytogenes for 40 min, unbound bacteria were washed away, and the cells were resuspended in medium containing gentamicin and incubated for 19 h. The cells were fixed and stained for F-actin with FITC phalloidin. The bacteria were revealed by the technique of differential immunostaining to distinguish between extracellular (green and red) and intracellular (only red) bacteria. Panels A, C, and E show three different fields in which Listeria cells are clearly seen projecting away from astrocytes or microglial cells. Typical Listeria actin tails are indicated by arrows. Panels B, D, and F show the labeling of the bacteria in red. (G and H) J774 macrophages previously infected with L. monocytogenes were cultured with primary glial cell cultures for 19 h in a cell culture medium containing gentamicin to kill extracellular listeriae. The cells were fixed and stained for F-actin, and the presence of intracellular bacteria was determined by differential immunofluorescence. Panel G shows an infected macrophage near an astrocyte. Panel H shows the presence of intracellular L. monocytogenes in an astrocyte (thick arrows). Scale bar, 5 μm.
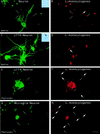
FIG. 4
Entry of L. monocytogenes into cultured neurons. (A and B) Neurons were incubated with L. monocytogenes for 40 min, unbound bacteria were washed away, and the cells were resuspended in medium containing gentamicin and incubated for 2 h. The cells were fixed and stained with a MAb raised against MAP-2A (panel A) and against the bacterial pathogen. Extracellular bacteria are indicated by thin arrows and are both green and red, whereas intracellular bacteria indicated by thick arrows are only red. The unique intracellular bacterium that we found in neurons is shown in panel B. Insets: phase-contrast microscopy of primary cultures of neurons infected with L. monocytogenes (A) and of neuron-infected J774 macrophage cocultures (B). The black arrow shows a macrophage adjacent to a neuron. (C through F) J774 macrophages previously infected with L. monocytogenes were cultured with neurons for 19 h in tissue culture medium containing gentamicin. The cells were fixed and stained with a MAb raised against MAP-2A (panel C) or stained for F-actin (panel E), and the presence of intracellular bacteria was examined by differential immunofluorescence. Panel C shows neurons. Panel D shows the presence of two intracellular L. monocytogenes in a neuron. Panel E shows an infected macrophage and the actin network of the neurons. Panel F shows the presence of intracellular L. monocytogenes cells in neurons close to the infected macrophage. (G and H) Cultured neurons were infected with L. monocytogenes for 40 min, washed, and incubated for 15 h in L15 complete medium containing gentamicin. Cells were fixed and stained for F-actin, and the presence of intracellular bacteria was determined by differential immunofluorescence. Panel G shows a microglial cell in the neuronal monolayers. In the neuronal processes located in the vicinity of the infected microglial cell, intracellular bacteria, indicated by thick arrows in panel H, were detected. Scale bar, 10 μm.
Similar articles
- Infection of murine fetal brain cell cultures with Listeria monocytogenes.
Peters M, Hewicker-Trautwein M. Peters M, et al. Vet Microbiol. 1994 Jul;41(1-2):19-28. doi: 10.1016/0378-1135(94)90132-5. Vet Microbiol. 1994. PMID: 7801522 - Studies on the cell tropism of Listeria monocytogenes in ovine fetal brain cell cultures.
Peters M, Hewicker-Trautwein M. Peters M, et al. Vet Microbiol. 1996 Apr;49(3-4):169-79. doi: 10.1016/0378-1135(95)00185-9. Vet Microbiol. 1996. PMID: 8734635 - Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua.
Geginat G, Nichterlein T, Kretschmar M, Schenk S, Hof H, Lalic-Mülthaler M, Goebel W, Bubert A. Geginat G, et al. J Immunol. 1999 Apr 15;162(8):4781-9. J Immunol. 1999. PMID: 10202020 - Axonal transport of Listeria monocytogenes and nerve-cell-induced bacterial killing.
Dons L, Jin Y, Kristensson K, Rottenberg ME. Dons L, et al. J Neurosci Res. 2007 Sep;85(12):2529-37. doi: 10.1002/jnr.21256. J Neurosci Res. 2007. PMID: 17387705 Review. - A review of the ecology, genomics, and stress response of Listeria innocua and Listeria monocytogenes.
Milillo SR, Friedly EC, Saldivar JC, Muthaiyan A, O'Bryan C, Crandall PG, Johnson MG, Ricke SC. Milillo SR, et al. Crit Rev Food Sci Nutr. 2012;52(8):712-25. doi: 10.1080/10408398.2010.507909. Crit Rev Food Sci Nutr. 2012. PMID: 22591342 Review.
Cited by
- Murinization of internalin extends its receptor repertoire, altering Listeria monocytogenes cell tropism and host responses.
Tsai YH, Disson O, Bierne H, Lecuit M. Tsai YH, et al. PLoS Pathog. 2013;9(5):e1003381. doi: 10.1371/journal.ppat.1003381. Epub 2013 May 30. PLoS Pathog. 2013. PMID: 23737746 Free PMC article. - Bovine neutrophil chemotaxis to Listeria monocytogenes in neurolisteriosis depends on microglia-released rather than bacterial factors.
Bagatella S, Haghayegh Jahromi N, Monney C, Polidori M, Gall FM, Marchionatti E, Serra F, Riedl R, Engelhardt B, Oevermann A. Bagatella S, et al. J Neuroinflammation. 2022 Dec 16;19(1):304. doi: 10.1186/s12974-022-02653-1. J Neuroinflammation. 2022. PMID: 36527076 Free PMC article. - Single-cell techniques using chromosomally tagged fluorescent bacteria to study Listeria monocytogenes infection processes.
Balestrino D, Hamon MA, Dortet L, Nahori MA, Pizarro-Cerda J, Alignani D, Dussurget O, Cossart P, Toledo-Arana A. Balestrino D, et al. Appl Environ Microbiol. 2010 Jun;76(11):3625-36. doi: 10.1128/AEM.02612-09. Epub 2010 Apr 2. Appl Environ Microbiol. 2010. PMID: 20363781 Free PMC article. - Listeria pathogenesis and molecular virulence determinants.
Vázquez-Boland JA, Kuhn M, Berche P, Chakraborty T, Domínguez-Bernal G, Goebel W, González-Zorn B, Wehland J, Kreft J. Vázquez-Boland JA, et al. Clin Microbiol Rev. 2001 Jul;14(3):584-640. doi: 10.1128/CMR.14.3.584-640.2001. Clin Microbiol Rev. 2001. PMID: 11432815 Free PMC article. Review. - Hofbauer Cells Spread Listeria monocytogenes among Placental Cells and Undergo Pro-Inflammatory Reprogramming while Retaining Production of Tolerogenic Factors.
Azari S, Johnson LJ, Webb A, Kozlowski SM, Zhang X, Rood K, Amer A, Seveau S. Azari S, et al. mBio. 2021 Aug 31;12(4):e0184921. doi: 10.1128/mBio.01849-21. Epub 2021 Aug 17. mBio. 2021. PMID: 34399615 Free PMC article.
References
- Barlow R M, McGorum B. Ovine listerial encephalitis: analysis, hypothesis and synthesis. Vet Rec. 1985;116:233–236. - PubMed
- Berche P. Bacteremia is required for invasion of the murine central nervous system by Listeria monocytogenes. Microb Pathog. 1995;18:323–336. - PubMed
- Camu W, Bloch-Gallego E, Henderson C E. Purification of spinal motoneurons from chicken and rat embryos by immunospanning. Neuroprotocols. 1993;2:191–199.
MeSH terms
LinkOut - more resources
Full Text Sources