Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells - PubMed (original) (raw)
Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells
P Krippeit-Drews et al. J Physiol. 1999.
Abstract
1. We have reported previously that in mouse pancreatic beta-cells H2O2 hyperpolarizes the membrane and increases the ATP-sensitive K+ current recorded in the perforated patch configuration of the patch-clamp technique. The present study was undertaken to elucidate the underlying mechanisms. 2. The intracellular ATP concentration measured by chemoluminescence was reduced by H2O2. The ADP concentration increased in parallel during the first 10 min, resulting in a pronounced decrease in the ATP/ADP ratio. 3. Consistent with these results, glucose-stimulated insulin secretion from isolated islets was inhibited by H2O2. 4. Membrane hyperpolarization measured with intracellular microelectrodes in intact islets and inhibition of insulin secretion were counteracted by tolbutamide, indicating that the channels are still responsive to inhibitors and that the ATP concentration is not too low to trigger exocytosis. However, the sensitivity of the beta-cells to tolbutamide was reduced after treatment with H2O2. 5. H2O2 increased the intracellular Ca2+ activity ([Ca2+]i) in a biphasic manner. A first transient rise in [Ca2+]i due to mobilization of Ca2+ from intracellular stores was followed by a sustained increase, which was at least partly dependent on Ca2+ influx. The first phase seems to reflect Ca2+ mobilization from mitochondria. 6. Our results demonstrate that H2O2 interferes with glucose metabolism, which influences the membrane potential and ATP-sensitive K+ current via the intracellular concentration of ATP. These events finally lead to an inhibition of insulin secretion despite an increase in [Ca2+]i.
Figures
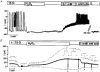
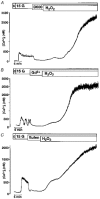
Figure 1. H2O2 hyperpolarizes the membrane potential by increasing the ATP-sensitive K+ current
A, effects of 1 mM H2O2 and 0.1 and 1 mM tolbutamide (tolb) on the membrane potential of mouse pancreatic β-cells in the presence of 15 mM glucose (15 G). The H2O2-induced hyperpolarization was irreversible but could be counteracted by inhibiting the ATP-sensitive K+ current with tolbutamide. The record is representative of four experiments with similar results. B, ATP-sensitive K+ current monitored in the perforated-patch mode in the presence of 15 mM glucose (15 G). The holding potential was -70 mV (continuous trace) and every 15 s 300 ms voltage steps to -80 and -60 mV (lower and upper dashed traces, respectively) were applied. H2O2 dramatically increased the current. Again the irreversible effect of H2O2 was at least partly counteracted by tolbutamide. The insets show the corresponding currents on an extended time scale. At the points marked by asterisks the amplifier was switched from voltage-clamp to current-clamp mode and the membrane potential was registered. The record is representative of five experiments with similar results.
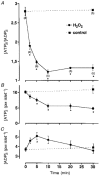
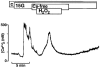
Figure 2. H2O2 diminishes intracellular ATP content
A, mean changes in the ATP/ADP ratio monitored with isolated islets after addition of 1 mM H2O2in the presence of 15 mM glucose. Numbers of experiments (n) given in parentheses below the symbols in A also apply to corresponding symbols in B and C. *Values significantly different from controls. B and C, H2O2 induced changes in intracellular ATP (B) and ADP (C) content. Note that the H2O2 effect is biphasic: within the first 5 min the loss in ATP coincided with an increase in ADP whereas afterwards the concentration of both nucleotides decreased.
Figure 3. H2O2 decreases the insulin secretion from isolated mouse islets
Effects of 1 mM H2O2 and 1 mM tolbutamide (tolb) on insulin release from perifused mouse islets. Switching from 3 to 15 mM glucose (3 G, 15 G) elicited the response of the islets to glucose. H2O2 irreversibly inhibited glucose-stimulated insulin secretion, an effect which was blunted by tolbutamide. Values are means ±
s.e.m.
of four experiments. Control experiments without H2O2 and tolbutamide are represented by the dashed line.
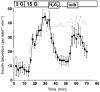
Figure 4. Effect of H2O2 on [Ca2+]i
Switching the extracellular glucose concentration from 0.5 mM (c = control) to 15 mM (15 G) led to an increase in [Ca2+]i. The subsequent addition of 1 mM H2O2 led to a marked drop in [Ca2+]i followed by a biphasic increase in [Ca2+]i, a first transient phase (see arrowhead) was followed by a large sustained increase. At the time intervals marked by the hatched bars Ca2+ was removed from the extracellular bath solution and 1 mM EGTA was added. After a steady-state level of [Ca2+]i had been reached, the Ca2+ ionophore ionomycin (1 μ
m
) was applied in the absence of extracellular Ca2+. The experiment shown is representative of five with similar results.
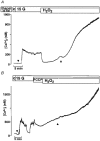
Figure 5. Influence of D600, Gd3+ and flufenamate on H2O2-induced changes in [Ca2+]i
In all experiments [Ca2+]i was first increased by switching from 0.5 mM glucose (c = control) to 15 mM glucose (15 G) in the bath solution. Neither the L-type Ca2+ channel blocker D600 (100 μ
m
; A) nor the inhibitors of non-selective cation channels and the Ca2+ release-activated Ca2+ current _I_crac, Gd3+ (100 μ
m
; B) or flufenamate (flufen, 100 μ
m
; C), suppressed the second marked rise in [Ca2+]i induced by 1 mM H2O2. Each experiment presented in this figure is representative of four experiments with similar results.
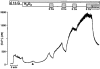
Figure 6. Effect of H2O2 on [Ca2+]i in Ca2+-free solution
Raising the glucose concentration from 0.5 mM (c = control) to 15 mM (15 G) increased [Ca2+]i. A subsequent switch to Ca2+-free solution (1 mM EGTA) restored [Ca2+]i to control values. Under these conditions the addition of 1 mM H2O2 led to a transient increase in [Ca2+]i, pointing to Ca2+ release from intracellular sites. The experiment shown is representative of nine with similar results.
Figure 7. Effect of the Ca2+ pump inhibitor thapsigargin and the mitochondria uncoupler FCCP on the first phase increase in [Ca2+]i induced by H2O2
A, the cells were pre-incubated for 30 min with 1 μ
m
thapsigargin. The effectiveness of the treatment is demonstrated by the complete suppression of the first initial decrease in [Ca2+]i after increasing the glucose concentration from 0.5 to 15 mM (compare traces at ▾ in A and B). Ca2+ store depletion by thapsigargin did not prevent the first rise in [Ca2+]i induced by H2O2 (▴). The experiment shown is representative of four with similar results. B, addition of 100 μ
m
FCCP in the presence of 15 mM glucose suppressed the first phase of the H2O2-evoked rise in [Ca2+]i (compare traces at ▴ in A and B). The experiment shown is representative of five with similar results.
Similar articles
- Involvement of ATP-sensitive K+ channels in free radical-mediated inhibition of insulin secretion in rat pancreatic beta-cells.
Nakazaki M, Kakei M, Koriyama N, Tanaka H. Nakazaki M, et al. Diabetes. 1995 Aug;44(8):878-83. doi: 10.2337/diab.44.8.878. Diabetes. 1995. PMID: 7621991 - Glucose regulation of insulin secretion independent of the opening or closure of adenosine triphosphate-sensitive K+ channels in beta cells.
Sato Y, Anello M, Henquin JC. Sato Y, et al. Endocrinology. 1999 May;140(5):2252-7. doi: 10.1210/endo.140.5.6729. Endocrinology. 1999. PMID: 10218978 - Tolbutamide and diazoxide influence insulin secretion by changing the concentration but not the action of cytoplasmic Ca2+ in beta-cells.
Mariot P, Gilon P, Nenquin M, Henquin JC. Mariot P, et al. Diabetes. 1998 Mar;47(3):365-73. doi: 10.2337/diabetes.47.3.365. Diabetes. 1998. PMID: 9519741 - Triggering and amplifying pathways of regulation of insulin secretion by glucose.
Henquin JC. Henquin JC. Diabetes. 2000 Nov;49(11):1751-60. doi: 10.2337/diabetes.49.11.1751. Diabetes. 2000. PMID: 11078440 Review. - Potassium channels of the insulin-secreting B cell.
Petit P, Loubatières-Mariani MM. Petit P, et al. Fundam Clin Pharmacol. 1992;6(3):123-34. doi: 10.1111/j.1472-8206.1992.tb00103.x. Fundam Clin Pharmacol. 1992. PMID: 1628875 Review.
Cited by
- Contrasting effects of alloxan on islets and single mouse pancreatic beta-cells.
Drews G, Krämer C, Düfer M, Krippeit-Drews P. Drews G, et al. Biochem J. 2000 Dec 1;352 Pt 2(Pt 2):389-97. Biochem J. 2000. PMID: 11085932 Free PMC article. - The glial antioxidant network and neuronal ascorbate: protective yet permissive for H(2)O(2) signaling.
Avshalumov MV, MacGregor DG, Sehgal LM, Rice ME. Avshalumov MV, et al. Neuron Glia Biol. 2004 Nov;1(4):365-76. doi: 10.1017/S1740925X05000311. Neuron Glia Biol. 2004. PMID: 18292802 Free PMC article. - Hydrogen peroxide activates calcium influx in human neutrophils.
Giambelluca MS, Gende OA. Giambelluca MS, et al. Mol Cell Biochem. 2008 Feb;309(1-2):151-6. doi: 10.1007/s11010-007-9653-9. Epub 2007 Nov 16. Mol Cell Biochem. 2008. PMID: 18008137 - Cooked common beans (Phaseolus vulgaris) protect against β-cell damage in streptozotocin-induced diabetic rats.
Hernández-Saavedra D, Mendoza-Sánchez M, Hernández-Montiel HL, Guzmán-Maldonado HS, Loarca-Piña GF, Salgado LM, Reynoso-Camacho R. Hernández-Saavedra D, et al. Plant Foods Hum Nutr. 2013 Jun;68(2):207-12. doi: 10.1007/s11130-013-0353-1. Plant Foods Hum Nutr. 2013. PMID: 23595343
References
- Ammon HPT, Hägele R, Youssif N, Eujen R, El-Amri N. A possible role of intracellular and membrane thiols of rat pancreatic islets in calcium uptake and insulin release. Endocrinology. 1983;112:720–726. - PubMed
- Brodie AE, Reed DJ. Reversible oxidation of glyceraldehyde 3-phosphate dehydrogenase thiols in human lung carcinoma cells by hydrogen peroxide. Biochemical and Biophysical Research Communications. 1987;148:120–125. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous